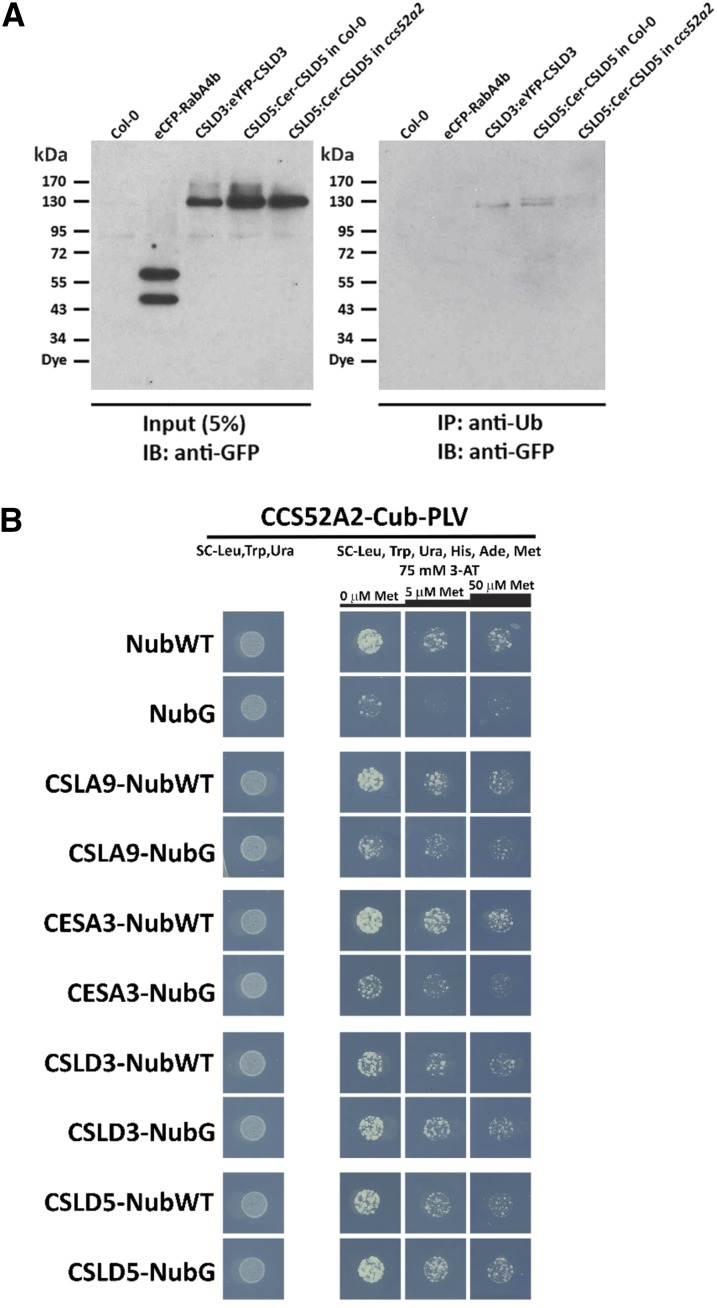
Figure 7.
CSLD Proteins Are Ubiquitin-Modified and Interact with the APC Activator Protein CCS52A2.
(A) Immunoprecipitation of ubiquitin-modified Arabidopsis proteins. Total proteins isolated from 5-d-old seedlings expressing eCFP-RabA4b, EYFP-CSLD3, or Cerulean-CSLD5 either in wild-type (Col-0) or ccs52a2 mutant backgrounds were subjected to immunoprecipitation with anti-Ub antibodies. Total proteins equivalent to 5% of input or antiubiquitin (anti-Ub)-immunoprecipitated proteins were separated by SDS-PAGE and fluorescently tagged proteins were detected by immunoblotting with anti-GFP antibodies.
(B) Detection of CCS52A2-CSLD interaction by split-ubiquitin yeast two-hybrid assay. Diploid yeast coexpressing CCS52A2-Cub-PLV bait constructs and various NubWT/NubG prey constructs. CCS52A2 was fused with Cub-PLV tag driven by a Met-repressible promoter. CSLA9, CESA3, CSLD3, and CSLD5 proteins were C-terminal tagged with both NubWT and NubG, respectively. Yeast were grown on vector-selective plates (SC-Leu, -Trp, -Ura) and interaction-selective plates (75 mM 3-AT, SC-Leu, -Trp, -Ura, -Ade, -His, -Met) with increasing Met concentrations (0, 5, and 50 µM Met). Yeast growth was recorded after 20 h for the vector-selective plates and 60 h for interaction-selective plates.