Abstract
Honey polyphenols have been studied with the objective of relating honeys to their floral sources. Initially synthesized by plant, these polyphenols can be found in the plant’s nectar, which are collected by bees, which convert the nectar into honey. Consequently, polyphenols constitute minor components of honey. The development of a solid-phase extraction method for honey polyphenols is presented in this study. The technique employs Amberlite XAD-2 adsorbent and was tested on monofloral honeys from six different plants: acacia, chestnut, eucalyptus, thyme, sunflower, and wild carrot. Analyses were performed using high-performance liquid chromatography coupled with UV detection and mass spectrometry. Several phenolic acids and flavonoids were identified: caffeic and p-coumaric acids, quercetin, kaempferol, naringenin, chrysin, and pinocembrin. Generally, the quantity of a given polyphenol in the honey was around 0.2 mg/100 g of honey, except for chestnut honey, which contained around 3.0 mg of p-coumaric acid/100 g of honey. Analyses highlighted significant formation of cis isomers for phenolic acids during the extraction despite protection from light.
Keywords: polyphenols, honey, solid-phase extraction, isomerization, chromatography
Introduction
When analyzing and studying the therapeutic properties of beehive products, modern science has made it possible to specify their medical significance as bactericidal, bacteriostatic, antiviral, antioxidant, anti-inflammatory, and antitumoral.1–7 The healing properties of honey largely depend on the floral source that nourishes the honeybees. Recent research in nectar chemistry shows us that nectar is more than water and sugars thanks to minor components which possess a wide range of bioactivities. The biochemical functions and different substances of nectar act as protection from microbial infestation through a novel biochemical pathway called the “Nectar Redox Cycle.”8–14 In order to identify the floral source of honeys, several chemical markers from nectar, including polyphenols, were proposed.
Polyphenols, meaning “substances which possess an aromatic ring bearing one (phenols) or more (polyphenols) hydroxyl substituents,”15 are a family of molecules that can be divided into several groups including phenolic acids, flavonoids, stilbenes, and lignans.16 More importantly in this context, they occur naturally as secondary plant metabolites.
Honey also contains polyphenols, mainly flavonoids, phenolic acids, and their derivatives.17–19 These compounds are derived from several sources such as nectar, pollen, honeydew, and propolis.17,20
Polyphenols are mainly present as derivatives of glycosides in the plant kingdom. These derivatives are often transformed into their aglycone form in honey due to bee enzymes like glucosidase, which acts on glucosides21,22 while leaving other glycosides (such as rhamnosides) unaffected.23
Nectar polyphenols are a common subject of study because they represent a promising way to link honey with its floral origin.19,24,25 Several works have already identified certain polyphenols as floral markers in honey. For instance, hesperitin, kaempferol, and quercetin have been shown to act as markers for honey from citrus, rosemary, and sunflower, respectively. Similarly, kaempferol rhamnosides and rhamnosylglucosides have been proposed as markers for acacia honey. Finally, ellagic, phenylactic, and mandelic acids are potential markers for heather honey; and caffeic, p-coumaric, and ferulic acids are potential markers for chestnut honey.24,25
Many authors have also reported the presence of honey in propolis polyphenols like pinocembrin, pinobanksin, galangin, chrysin, and tectochrysin.20,25 Propolis is a resinous substance collected by bees from plant buds,26 primarily occurring in exudates from members of the Populus, Betula, Pinus, Prunus, and Alnus families with a geographical range spanning Europe, North America, and non-tropical regions of Asia.27 Since the compounds found in propolis can vary greatly depending on the region in which they were cultivated,28 an analysis of the relative abundance of propolis-derived polyphenols could indicate the geographic source of a honey sample.
Extraction of polyphenols from honey is generally accomplished using either liquid–liquid extraction (LLE) or solid-phase extraction (SPE). In both methods, the first step is to separate the sugars, which make up the great majority of the honey mass, from the less abundant but more interesting honey components. This separation must occur without heating the samples above 50°C due to the heat sensitivity of polyphenols.19,29 In the case of LLE, solvents like ethyl acetate, ethanol, or chloroform are often used for this purpose,17,29,30 while SPE generally involves dissolution of honey in a pH 2 aqueous hydrochloric acid solution. The mixture is then passed through octadecyl-grafted resins or polymeric resins like Amberlite XAD-2, Oasis HLB, or Strata-X on which the polyphenols are adsorbed.18,19,31 The extract is then evaporated to dryness under vacuum at 40°C and reconstituted in methanol or a water–methanol mixture. Further purification with diethyl ether is occasionally required.17,29,31,32 The product is then characterized using high-performance liquid chromatography (or HPLC) coupled with UV detection, mass spectrometry, or a combination of the two.29
This work demonstrates a precise method by which the polyphenol content of a honey sample may be studied. The method was performed on six samples of honey, each derived from a different monofloral origin. Contrary to preceding research on the subject, this work attempts to explicitly identify and control as many potential sources of error as possible. In brief, the polyphenols are first separated from honey sugars through a solid-phase extraction. After extraction, they are injected into an HPLC and characterized by correlating their elution time, UV spectrum, and mass spectrum. It is expected that this document will serve as a guide for subsequent analysis of the chemical composition of honeys. Finally, this work describes the characterization of cis isomers of some polyphenols of honey, a phenomenon that has not yet been reported in literature and which could be responsible for inaccurate characterisation of honey samples.
Materials and Methods
Materials
The following organic monofloral honey samples of Italian origin were purchased from Nectar & Co, Fernelmont, Belgium: chestnut honey from Piedmont (batch L2013-CH-38-1), sunflower honey from Emilia Romagna (batch L2013-TO-31.32-1), eucalyptus honey from Sardinia (batch L2013-EU-55.56-1), and thyme (batch L2014-TH-1.2-1) and wild carrot honey from Syracuse (batch L2013-CA- 107.108-1). Acacia monofloral honey from Romania (batch r0000001a) was provided by BeeOdiversity, Ixelles, Belgium. The six samples were preserved at 4°C in the dark.
Acetonitrile (99.85% HPLC and >99.9% LC-MS) and methanol were provided by Scharlau (Scharlab, S.L, Barcelona, Spain). Formic acid (ULC/MS 99%) was provided by Biosolve Chemical Inc, Dieuze, France, and acetic acid was provided by VWR chemicals, Radnor, Pennsylvania, USA.
Caffeic acid (>98% HPLC), chlorogenic acid (>95% titration), ellagic acid (>95% HPLC), gallic acid (97,5-102.5%), p-coumaric acid (>98% HPLC), trans-ferulic acid (99.4%), rutin hydrate (>94% HPLC), kaempferol (>90% HPLC), naringenin (>95%), quercetin (>95% HPLC), and chrysin (>98% HPLC) were obtained from Sigma-Aldrich Inc, Saint-Louis, Missouri, USA.
Sugar standards composed of d(−)-fructose (>99.0% HPLC), d(+)-glucose (>99% GC), d(+)-maltose monohydrate (>99% HPLC), d(+)-sucrose (>99.5% GC), and d(+)-trehalose dihydrate (n.d.) were provided by Sigma-Aldrich Inc.
Amberlite XAD-2 resin (mean pore size: 90 Å, particle size: 20–60 mesh) was also provided by Sigma-Aldrich Inc.
Method for extracting and characterizing polyphenols in honey
Extraction protocol
Solid-phase extraction was conducted in a glass column fitted with an opening valve and a fritted glass support (pore size: 100–160 μm). The column (diameter: 2 cm) was packed with 30 g of Amberlite XAD-2 resin. The resin was prepared according to the supplier’s recommendations. Extraction steps are presented in Figure 1. The column and collected fractions were protected from light. Sugars were eliminated with hydrochloric acid solution and water washes. Polyphenols were desorbed with methanol, then dried at 40°C using a Buchi Rotavapor, and protected from light. Because this fraction contained some residual water, evaporation was conducted in two steps: a first one at 190 mbar and a second one at 50 mbar. When no more water could be evaporated, the residue (2–3 mL) was made up to 5 mL with a mixture of water and methanol (20:80 v/v). This diluted residue was subsequently injected to a high-performance liquid chromatography column coupled with diode array detection (HPLC-DAD) and to liquid chromatography column coupled with UV detection and mass spectrometry (LC-UV-MS). The “extracted phenols” test, described elsewhere in this article, was also performed on these solutions to determine the total quantity of polyphenols extracted from a given sample, as the name suggests. All analyses were performed in triplicates.
Figure 1.
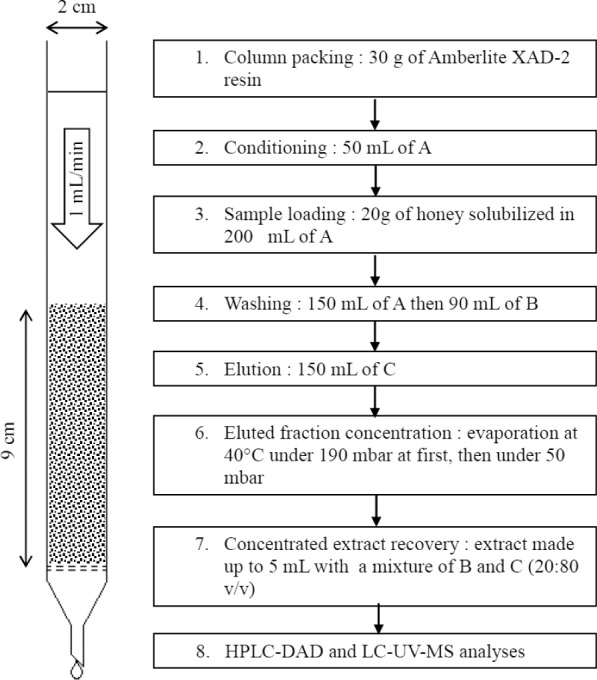
Honey polyphenols extraction steps. Used solvents are a hydrochloric acid solution at pH 2 (A), distilled water (B), and methanol (C).
HPLC-DAD
In order to obtain UV spectra for compounds of interest, an HPLC Alliance 2690 (Waters) device coupled with a Waters 996 PDA detector was used. A Zorbax 300 sb-C18 (3.5 μm, 4.6 × 150 mm) from Agilent was mounted on the chromatographic module and the temperature was set to 25°C.
A binary mobile phase was used, consisting of a first solvent (A), distilled water with acetic acid (0.5%) and a second one (B), acetonitrile with acetic acid (0.5%). The total flow rate was 1 mL/min and the injection volume was 15 μL. The gradient began with 100% A. This proportion was held for 5 minutes, after which the gradient decreased to 85% A at 10 minutes, then steadily to 65% A at 30 minutes, and then to 50% at 35 minutes, and finally, cut off to 0% at 36 minutes. This ratio was held until 40 minutes. After that, the proportion of A increased to 100% at 41 minutes and held there until 46 minutes.
By scanning from 200 to 400 nm, calibration curves for gallic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, quercetin, kaempferol, and chrysin prepared in a water–methanol mixture (20:80 v/v) were constructed. After identification, this technique was also used for quantification at 280 and 320 nm with external calibration. Parameters of the HPLC-DAD method are summarized in Supplementary Table 1.
LC-UV-MS
LC-UV-MS analyses were carried out using a 1100 Series chromatographic device from Agilent Technologies coupled with UV detector (1100 Series Agilent Technologies) and mass spectrometer (Esquire HCT, Bruker Daltonics). For this experiment, an Eclipse XDB C18 column of Agilent was used (3.5 μm, 2.1 × 150 mm). The mobile phase employed two solvents, a solvent “A” consisting of acetonitrile with 0.1% formic acid and a solvent “B” composed of Milli-Q water, also with 0.1% formic acid. The overall flow rate was 0.2 mL/min with a column temperature of 30°C. The composition used for this experiment started at 85%. The proportion of A in the stream then steadily decreased until it reached 40% after 30 minutes. Between 30 and 35 minutes, the proportion of solvent A in the stream then decreased from 40% to 20%. After the 35th minute, the proportion of A then increased to reach 85% at 50 minutes. Finally, this ratio was held for 10 minutes. The injection volume was 5 μL.
UV-detection was conducted at 280 and 320 nm according to a method proposed by Proestos and Komaitis (2013).33
Mass spectrometry was conducted using electrospray ionization, in this case with an ion trap device in the negative mode. Nebulization gas was nitrogen with a flow rate of 9 L/min and a nebulization pressure of 40 psi. Dry gas temperature was set on 365°C. The m/z range was between 100 and 1,000. Identification was based on retention time, m/z ratio, and UV absorption. Retention time and m/z ratio of phenolic compounds can be found in Supplementary Table 2. LC-UV-MS method parameters are summarized in Supplementary Table 3.
Extracted phenols
The global amount of extracted phenolic compounds, referred to as “extracted phenols,” was assessed using Folin–Ciocalteu reagent according to a protocol described by Vanderghem et al (2014).38 Analyses were performed with a UV-1800 Shimadzu spectrophotometer at 750 nm by external calibration using gallic acid solubilized in a water–methanol mixture (20:80 v/v). Results are therefore expressed in gallic acid equivalents (GAE) per 100 g of honey. Analyzed solutions consist of 100 μL of honey extract added to 500 μL of Folin Reagent and 2 mL of Na2CO3 (20 wt-% in water). Absorbance was measured after 30 minutes at room temperature.
Validation experiments
One objective of this work was to explicitly characterize as much of the materials and methods as possible in order to more precisely quantify experimental error and to aid further development of methods for characterization of honey and similar mixtures.
“Artificial honey” as calibration standard
In order to more accurately assess the effectiveness of the separation processes used in this work, an “artificial honey” of a known composition was prepared according to a method proposed by Campone et al (2014).17 This artificial honey was intended to closely match the composition of a typical honey, in this case, acacia honey. To this end, analysis was performed in triplicate on the acacia monofloral honey from BeeOdiversity in accordance with the Harmonized Methods of the International Honey Commission.34 This analysis included determining the content of sugar, water, ash, protein, and gluconic acid, as well as the pH of the honey. The relevant characterization methods are described below.
The “artificial” honey comprised 6 g of glucose, 8 g of fructose, 0.8 g of sucrose, and 100 μg of a combination of polyphenols (gallic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, quercetin, kaempferol, and chrysin). These substances were solubilized in 200 mL of hydrochloric acid solution (pH 2) as well as honey samples before loading on the extraction column. Using this “artificial honey,” the efficiency of a given separation process can be easily evaluated by comparing the composition of a given outlet stream to the known composition of the inlet.
Determining sugar content in honeys
Sugars were analyzed by high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD). Manufactured by Thermo Scientific under the brand name Dionex, the device included three modules: an automatic sampler AS-AP, a chromatographic and detection module ICS-5000 + DC, and a pumps module ICS-5000 + SP. Identification and quantification were achieved by external calibration with standard solutions of d(−)-fructose, d(+)-glucose, d(+)-maltose monohydrate, d(+)-sucrose, and d(+)-trehalose dehydrate. A CarboPac PA 100 (4 × 250 mm) column from Thermo Scientific was employed. The injected sample volume was 25 μL and the column was heated to 35°C. Analyses were performed in gradient mode with two solvents: a 500 mmol/L NaOH solution (A) and Milli-Q water (B). Initially, solvent A constituted 30% of mobile phase. After 15 minutes, the proportion of solvent A began to increase at a steady rate until reaching 40% at 25 minutes, after which the proportion was then decreased until again reaching 30% at 30 minutes. The general flow rate was 1 mL/min.
Water content
Water content was determined by refractometry with a DIGIT-5890 ATC of VWR international. The procedure followed the recommendations of the Harmonised Methods of the International Honey Commission.34
Ash content
Ashes were analyzed by calcination in a Nabertherm muffle furnace (Controller B180). Samples were dried for 24 hours at 105°C beforehand. Then calcination was performed following a temperature ramp of 2 hours from 25°C to 575°C. This last temperature was maintained for 4 hours.
pH
Samples (2 g) were solubilized in exactly 20 mL of distilled water (previously boiled and cooled). pH was measured on this solution with a pH meter.
Gluconic acid
Gluconic acid content was determined by spectrophotometry (UV-1800 Shimadzu) following the protocol mentioned in the Megazyme test kit: d-gluconic acid/d-glucono-δ-lactone Assay procedure K-Gate 11/05.
Protein content
Protein content was evaluated using the Bradford method. Bovine serum albumin was used as standard according to the recommendations of Azeredo et al (2003).35 Analyses were conducted using the Bio-Rad “Quick Start Bradford Protein Assay.”
5-Hydroxymethylfurfural content
5-Hydroxymethylfurfural content (HMF) concentration was measured in honey in order to monitor degradation. Polyphenols are heat-sensitive molecules, and since HMF content increases with the intensity and duration of thermal treatments, it is a reliable indicator of degradation. To determine the concentration of HMF, honey samples (0.5 g) were dissolved in 5 mL of distilled water. After homogenization, sample solutions were filtered on polytetrafluoroethylene syringe filters (0.45 μm) and analyzed with high-performance liquid chromatography (Waters 2695) coupled with UV detection (Waters 2487 Dual λ Absorbance). The device was used in isocratic mode with a Zorbax 300 sb-C18 (4.6 × 150 mm; 3.5 μm) column at 30°C. The solvent consisted of a mixture of distilled water (89.1%), acetic acid (0.9%), and methanol (10%), delivered with a flow of 1 mL/min for 30 minutes. The injection volume was 20 μL and the detector was set to 284 nm.
Estimating polyphenol recovery yield
In order to calculate the efficiency of the extraction process, the recovery rate was determined for several polyphenols based on an extraction performed on “artificial” honey. This “artificial” honey was loaded on a column like the samples and the extraction process was followed as described in Section 2.2. The quantities of polyphenols in the artificial honey correspond in principle to those found in 20 g of honey (0.5 mg of each polyphenols/100 g of honey). This value can be inferred from literature that mentioned variable amounts of polyphenols from less than 0.01 mg to more than 1 mg/100 g of honey for each compound.17,24,36,37 After evaporation, the residues were made up to 10 mL with a mixture of water and methanol (20:80 v/v). These solutions were injected in HPLC-DAD for quantification of extracted polyphenols.
Determining elution time for SPE
This experiment was performed on artificial honey in order to determine the elution times of the polyphenols of interest. Extraction was conducted as described in Section 2.2 except that the elution was recovered in 10 separate fractions rather than simply collecting all the elution volume in one receptacle. Each fraction was evaporated at 40°C under 190 mbar. Residues were separately made up to 1 mL with a mixture of distilled water and methanol (20:80 v/v). The fractions were then analyzed with HPLC-DAD and LC-UV-MS.
Quantification of residual sugar post extraction
Concentrated extracts of each honey sample, including the artificial honey, were analyzed using the HPAEC-PAD described in Subsection 2.3.2 with the aim of evaluating residual sugars after the extraction described in Section 2.2.
Adsorption capacity of resin
No information could be found in literature or with the supplier about the adsorption capacity of resins used for this extraction. Consequently, tests were performed to ensure that the column capacity was sufficient for the intended extraction. 400 mL of a p-coumaric acid solution (100 mg/L) and 500 mL of a quercetin solution (50.4 mg/L) were separately passed through two extraction columns (prepared as in Section 2.2) with a flow of 1 mL/min. These solutions were made using aqueous hydrochloric acid calibrated to pH 2 as solvent. Fractions of 10 mL each were collected during the load. After loading the column, p-coumaric acid and quercetin were eluted with 200 mL of methanol at a flow rate of 1 mL/min. Again, 10 mL fractions were collected and analyzed with HPLC-DAD.
Results and Discussion
Acacia honey composition
The chemical species identified in Acacia honey accounted for about 95% of its total composition. The results are presented in Table 1 and compared with characterizations of honey reported in literature.
Table 1.
Acacia honey composition.
| COMPOUNDS | CONTENTS (%) |
|---|---|
| Glucose | 30.78 ± 0.20 |
| Fructose | 42.89 ± 1.99 |
| Sucrose | 3.88 ± 0.08 |
| Maltose | 1.17 ± 0.05 |
| Ashes | 0.06 ± 0.01 |
| Proteins (Bradford) | 0.02 ± 0.003 |
| Gluconic acid | 0.17 ± 0.004 |
| Water (refractometry) | 16 ± 1 |
| Total | 95 |
Sugar and water content corresponded well with results presented by Ball (2007)39 and Desmouli (2013).40 Ash content (0.06%) seemed quite low but remained between the extreme values of 0.02% and 1.03% cited by Crane (1975).41 A pH value of 4.16 was measured; this value seemed to match with values found by Desmouli (2013).40 However, the concentration of gluconic acid observed in this sample (0.17%) was quite small compared to that reported by Ball (2007),39 which was around 0.60%. Similarly, protein content (0.02%) was lower than what was reported by Chua et al (2013)42 from 0.1 to 0.5%. Finally, HMF was present at an average concentration of 3.94 ± 0.85 mg/kg of honey, indicating that the sample had not been overheated during the extraction. This quantity was far below the legal limit of 40.0 mg/kg in Belgium.
Polyphenol extraction method
Polyphenol recovery
Among polyphenols monitored in this experiment (gallic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, quercetin, kaempferol, and chrysin), gallic acid was not recovered, as no trace of this compound was found in artificial honey extract or in honey samples extracts. The molecule was probably too polar to stay adsorbed on the resin after washing with water. This phenomenon was also observed by Michalkiewicz et al (2008)31 who demonstrated loss of gallic acid before methanol elution. Obtained recoveries were 91.62% ± 3.10% for caffeic acid, 54.65% ± 14.04% for p-coumaric acid, 70.49% ± 12.11% for ferulic acid, 97.25% ± 13.98% for quercetin, 102.15% ± 10.49% for kaempferol, and 59.26% ± 17.05% for chrysin. In comparison, Michalkiewicz et al (2008)31 used the same absorbent resin (Amberlite XAD-2), and reported recoveries of approximately 90% for kaempferol, 50% for quercetin, 100% for p-coumaric acid, and 80% for caffeic acid. These values were obtained from standard solutions of each polyphenol in water. Due to the difference in composition between these samples and actual honey, it is likely that the recovery rates reported for the former are not a good guide for recovery rates to be expected from the latter. In fact, when the same authors loaded their column with spiked samples of honey, they were only able to recover 42% of the quercetin, 66% of the p-coumaric acid, and 27% of the caffeic acid from their samples. Pyrzynska et al (2009)29 also showed recoveries higher than 80% for caffeic acid, p-coumaric acid, and kaempferol, and lower than 60% for quercetin, but again these results were obtained with standard solutions, not with spiked samples or artificial honey.
It is also possible that our results underestimate the amount of polyphenol recovered. HPLC-DAD and LC-UV-MS analyses, shown in Figure 2, revealed secondary peaks occurring after each phenolic acid with the same mass as the preceding peak and UV spectra characteristic of phenolic acids. Referring to Figure 2, one finds peaks at 12.27, 13.96, and 14.84 minutes corresponding to caffeic acid, p-coumaric acid, and ferulic acid respectively, and peaks at 22.21, 26.16, and 33.23 minutes correspond to quercetin, kaempferol and chrysin respectively. They were identified by comparison with the spectra produced from standard solutions in HPLC-DAD and LC-UV-MS. Using the mass spectra obtained from the standard solutions, it was also possible to identify the unidentified peaks based on their m/z ratio: for instance, the peak at 12.60 minutes had a molecular weight of 179, corresponding to the molecular weight of caffeic acid. Likewise, the peak occurring at 14.21 minutes had an m/z ratio of 163, corresponding to p-coumaric acid, while the peak at 15.20 minutes had an m/z ratio of 193, corresponding to ferulic acid. The most probable hypothesis to explain these secondary peaks is a cis isomerization. Each phenolic acid standard consisted of the trans isomer of the compound, so the elution time of peaks recorded for the standard solutions was not representative of the cis isomers, which must have appeared during the extraction. Consequently, these isomers were not taken into account when the recovery fraction was calculated.
Figure 2.
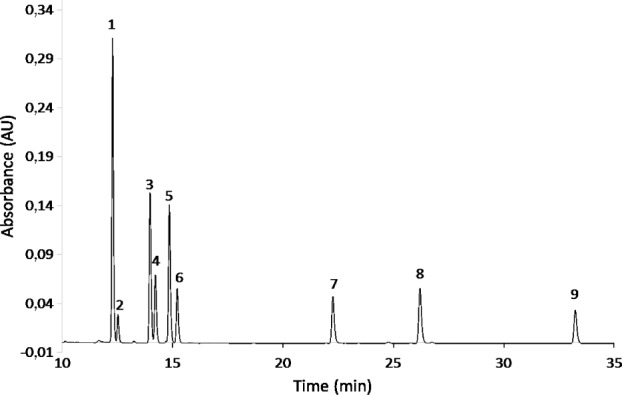
HPLC-DAD chromatogram of “artificial” honey extract at 320 nm. (1: trans caffeic acid, 2: cis-caffeic acid, 3: trans-p-coumaric acid, 4: cis-p-coumaric acid, 5: trans-ferulic acid, 6: cis-ferulic acid, 7: quercetin, 8: kaempferol, 9: chrysin).
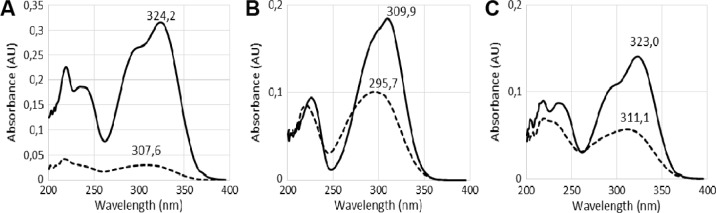
UV spectra also support the presence of cis isomers. Caccamese and Azzolina (1979)43 mentioned a hypsochromic shift for the cis form. This shift was confirmed by UV spectra of the analyzed phenolic acids (Fig. 3) since absorption maxima of cis forms are at shorter wavelength than those of trans forms. Those spectra correspond to the six first peaks of Figure 2. For cis- and trans-ferulic acids, UV spectra shown in Figure 3 are very similar to those obtained by Caccamese and Azzolina (1979).43
Figure 3.
UV spectra of trans (continuous line) and cis (interrupted line) forms of caffeic (A), p-coumaric (B), and ferulic (C) acids with absorption maxima (nm).
Isomerization causes were investigated in literature. According to Voncina et al (2009),44 trans-p-coumaric acid and trans-ferulic acid in methanol underwent cis isomerization during light exposure at ambient temperature after a few hours. However, all our extraction glassware was protected from light and temperature never exceeded 40°C during the concentration step. In conclusion, results suggest that cis isomerization can occur even with very low light exposure or that light is not necessarily the main isomerization cause. Simple exposure to ambient temperature in methanol or an unknown reaction with the adsorbent might be responsible for the isomerization, but further investigation is required.
SPE elution
Elution order and solvent volume were determined by collecting 10 elution fractions of 15 mL from a column loaded with “artificial” honey. The first fraction is mainly composed of water (from the dead volume of the column) and HPLC-DAD analysis did not reveal any compounds in this fraction. Phenolic acids, quercetin and kaempferol were detected in the second fraction, but this fraction still contained water. By time the second fraction was collected, all of the water initially present in the column had been flushed and the solvent flow was purely composed of methanol. The third fraction contained the same polyphenols as the second one, with the addition of chrysin. The fourth fraction contained caffeic acid, p-coumaric acid and chrysin. From the fifth to the eighth fraction, chrysin continued to be present, and nothing of interest was detected in fractions 9 and 10. In consequence, 120 mL of methanol were determined to be sufficient to desorb all the compounds discussed in this work. Some potentially useful flavonoids like pinocembrin are likely have more affinity for the adsorbent than chrysin and therefore would require more solvent elution in order to be extracted. As a precautionary measure, an elution volume of 150 mL of methanol was used.
Residual sugars
The quantity of residual sugars remaining in samples after extraction were analyzed in all concentrated honey extracts, in case they might be found to distort the elution time or UV spectra of polyphenols in the supposedly sugar-free samples. Glucose and fructose concentrations were found to vary from 79 to 390 mg/L. For the artificial honey extract, residual concentration for each sugar was lower than 150 mg/L. If the acacia honey composition is used as a reference, more than 99.96% of the glucose and 99.97% of the fructose initially present in the sample were extracted by the column. Residual sugar concentrations can be significant for minor component analysis like polyphenols. Interference can nevertheless be limited thanks to the absence of UV absorption of sugars at the wavelengths used to analyze polyphenols (280 and 320 nm).
Adsorption capacity
No information could be found about the adsorption capacity of Amberlite XAD-2, so this was evaluated using aqueous standard solutions of p-coumaric acid and quercetin as described in subsection 2.3.12 of the experimental method. During loading, the compounds were not detected in the outlet stream from the column, meaning p-coumaric acid and quercetin were properly adsorbed on the resin. Both compounds could then be quickly recovered by eluting with methanol—from the second fraction onward, the compounds could be detected in the elution stream. These tests showed that for 30 g of resin, the adsorption capacity is at least 1.3 mg/g for p-coumaric acid and 0.84 mg/g for quercetin, more than enough for honey samples.
Extracted phenols
Extracted phenols content, presented in Figure 4, varies significantly between honeys from different floral origins. Chestnut honey is the richest sample (17.3 mg GAE/100 g honey) while acacia honey seems rather poor in polyphenols (1.6 mg GAE/100 g honey). These results should be considered with caution because of possible interferences. Indeed, if tests performed in the laboratory demonstrated the absence of free monosaccharides interferences, amino acids like tyrosine could have some impact on the absorbance because of its aromatic structure.
Figure 4.
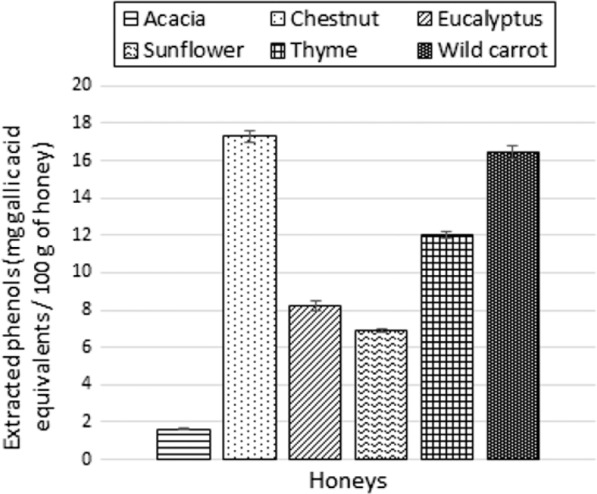
Extracted phenols (mg acid gallic equivalent/100 g of honey) for each honey samples.
Identification and quantification of polyphenols of interest
LC-UV chromatogram of sunflower honey extract is presented in Figure 5. Six substances were identified in sunflower honey were caffeic acid, p-coumaric acid, quercetin, kaempferol, chrysin, and pinocembrin. The identity of each compound was inferred from their m/z ratios (179, 163, 301, 285, 253, and 255, respectively), UV spectra, and corresponding with their retention times (6.2, 10.3, 19.3, 22.7, 29.1, and 29.9, respectively). Pinocembrin was identified from m/z ratio. The chromatograms of the other honeys are displayed in the supplementary data section (Supplementary Figs. 1–5).
Figure 5.
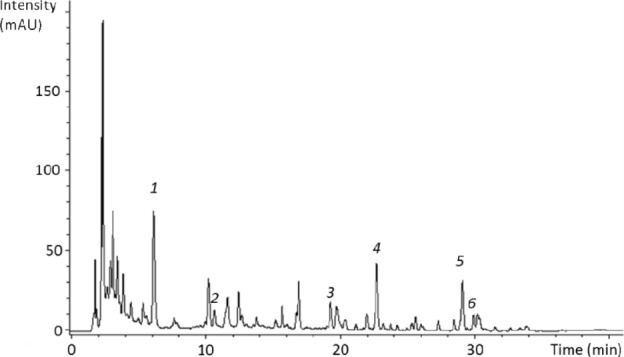
Sunflower honey extract chromatogram at 320 nm obtained with the LC-UV-MS method. (1: caffeic acid, 2: p-coumaric acid, 3: quercetin, 4: kaempferol, 5: chrysin, 6: pinocembrin).
The polyphenols identified in acacia honey were caffeic acid, p-coumaric acid, and chrysin. Each compound was identified from m/z ratios 179, 163, and 253, respectively and corresponding retention time (6.3, 10.4, and 29.1, respectively). p-Coumaric acid and chrysin were also identified by comparison of their UV spectra with those of standards. An unknown compound was found at a retention time of 11.7 minutes. Its UV spectrum was very similar to that of ferulic acid although m/z ratio was 199, which is too heavy to be ferulic acid. These observations suggest that it could be a polyphenol, although it could not be positively identified.
p-Coumaric acid, chrysin, and pinocembrine were identified in chestnut honey with m/z ratios 163, 253, and 255, respectively. p-Coumaric acid and chrysin were also found thanks to their retention time. But only p-coumaric acid showed a UV spectrum similar to that of the standard. For chrysin, the peak was too small to allow confirmation of the identity with UV spectrum. Another compound detected at 11.9 minutes and is supposed to be a polyphenol although its identity was not positively determined. The UV spectrum of this molecule showed two absorption maxima at 229.7 and 308.8 nm, a characteristic pattern of polyphenols. Moreover, its m/z ratio was 187.8, which is within the range of phenolic acid m/z ratio.
The three compounds identified in eucalyptus honey were caffeic acid, quercetin and kaempferol. The m/z ratios were 179, 301, and 285, respectively, and retention times were 6.4, 19.3, and 22.8 minutes, respectively, which corresponded to those of standards. Only quercetin’s identity could be confirmed from its UV spectrum. Again, an unidentified compound was found at a retention time of 16.1 minutes with an m/z ratio of 301 and UV absorption at 280 and 320 nm, suggesting that it could be a polyphenol and more specifically a flavonoid or a flavonoid glycoside.
p-Coumaric acid, naringenin, chrysin, and pinocembrin were identified in thyme honey. p-Coumaric acid, naringenin, and chrysin were determined from their m/z ratios (163, 271, and 253, respectively), retention times (10.1, 22.7, and 29.1 minutes, respectively), and UV spectra. Pinocembrin was again tentatively identified from its m/z ratio alone (255).
Caffeic acid, p-coumaric acid, chrysin, and pinocembrin were identified in wild carrot honey. Observed m/z ratios were 179, 163, 253, and 255, respectively, for the following retention times: 6.3, 10.3, 29.1, and 29.9 minutes, respectively. An unidentified compound already observed in chestnut honey was found at a retention time of 11.9 minutes with an m/z ratio of 188. Once again, the compound had strong UV absorption at 280 and 320 nm, suggesting a polyphenolic structure.
It should be noted that in each honey, a common substance with an m/z ratio of 488 was observed at a retention time of 2.3 minutes. Another unknown compound was also found in acacia and chestnut honey with an m/z ratio of 551 a retention time of 3.0 min. Both compounds absorbed strongly at 280 and 320 nm.
Table 2 displays the quantification of some polyphenols of interest with HPLC-DAD. The other compounds could not be quantified because they were outside calibration range or because no commercial standard was available.
Table 2.
Honey polyphenols quantified with the HPLC-DAD method.
| HONEYS | IDENTIFIED POLYPHENOLS | MEANS ± SD (mg/100 g de miel) |
|---|---|---|
| Acacia | p-coumaric acid | 0.077 ± 0.003 |
| Chestnut | p-coumaric acid | 2.952 ± 0.004 |
| Eucalyptus | Quercetin | 0.164 ± 0.007 |
| Sunflower | Caffeic acid | 0.242 ± 0.001 |
| p-coumaric acid | 0.107 ± 0.000 | |
| Quercetin | 0.276 ± 0.003 | |
| Kaempferol | 0.205 ± 0.003 | |
| Chrysin | 0.217 ± 0.002 | |
| Thyme | p-coumaric acid | 0.070 ± 0.000 |
| Wild carrot | p-coumaric acid | 0.223 ± 0.001 |
Table 2 shows that polyphenol content in honeys is generally around 0.2 mg/100 g. However, chestnut honey displayed a much higher content, reaching almost 3 mg/100 g.
Finally, it should be noted that since gallic acid is lost during the extraction due to its high polarity, some other potential markers like glycosides might not be extracted. Consequently, the potential for glycosides retention was investigated in literature. For example, Truchado et al (2008)23 demonstrated the potential of flavonol rhamnosides as floral markers for acacia honey. However, according to their chromatographic analyses, kaempferol glycosides retention times are ranged between those of caffeic acid and chrysin (the most and the least polar compounds extracted and identified with our technique respectively). This might reflect an intermediate polarity of some glycosides, which means that a part of these compounds could be extracted with our technique. Kato et al45 also showed that leptosin, another glycoside, could be used as a marker for manuka honey. Their isolation method with a styrene-divinylbenzen resin and the retention time of leptosin during chromatographic analyses suggest again an intermediate polarity for this compound and consequently a potential retention on our adsorbent. If our solid-phase extraction is not efficient for the most polar compounds as gallic acids, it is still useful for a wide range of phenolic substances. Michalkiewicz et al31 showed that no common adsorbent could extract all the “polarity spectrum” of phenolic compounds. If resins like Oasis HLB are more efficient for polar compounds (gallic acid can be retained), performances for less polar substances (like quercetin or kaempferol) are generally lower.
Conclusions
A method for identifying and quantifying polyphenols in honey is used to determine the polyphenol content in six honeys each originating from a different monofloral origin. The reliability and precision of this method is examined using a variety of experiments adapted from previous work on the analysis of honey. Ninety-five percent of the composition of an acacia honey sample was characterized, and an “artificial honey” standard was produced simulating the composition of the acacia honey. Since this standard effectively had the same composition as the honey samples examined, it could more reliably be used to determine the efficiency of the experimental apparatus.
Some polyphenols were recovered with almost no loss: for instance, quercetin and kaempferol were recovered at more than 90%. On the other hand, chrysin was recovered only at about 60%. This disparity might be explained by a stronger interaction between chrysin and the adsorbent, as demonstrated by the elution time for this species: as described in subsection 3.2.2, chrysin continued to be eluted from the SPE column for much longer than was the case for the other polyphenols. Finally, the extracted phenols were analyzed to determine the combined concentration of all phenols in a sample, a quantity that ranged from 2 to 17 mg GAE/100 g of honey.
UV and mass spectra revealed cis-isomerized analogs to the phenolic acids after extraction from the artificial honey. Cis isomerization was not observed for honey samples but this phenomenon should be considered for further studies about honey polyphenols. Considering this isomerization, the reported extraction yields (eg, 92%, 55%, and 70% for caffeic, p-coumaric, and ferulic acid, respectively) for trans isomers might underestimate the actual quantity of polyphenol recovered, since cis isomers of these polyphenols were not counted.
Identification and quantification of individual compounds of interest reveal significant diversity between honeys from different floral origins.
These results support the research on honey polyphenols as floral markers to link these products to their sources.
Supplementary Materials
Supplementary table 1. HPLC-DAD method.
Supplementary table 2. Retention time and m/z ratio of phenolic compounds in LC-UV-MS.
Supplementary table 3. LC-UV-MS method.
Supplementary figure 1. Acacia honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: caffeic acid, 2: p-coumaric acid, 3: chrysin).
Supplementary figure 2. Chestnut honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: p-coumaric acid, 2: chrysin, 3: pinocembrin).
Supplementary figure 3. Eucalyptus honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: caffeic acid, 2: quercetin, 3: kaempferol).
Supplementary figure 4. Thyme honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: p-coumaric acid, 2: naringenin, 3: chrysin, 4: pinocembrin).
Supplementary figure 5. Wild carrot honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: caffeic acid, 2: p-coumaric acid, 3: chrysin).
Acknowledgments
The authors would like to thank Virginie Byttebier, Alexandre Schandeler, Catherine Chemotti, and Isabelle Van De Vreken for the technical assistance, and BeeOdiversity, Ixelles, Belgium, for providing honey sample.
Footnotes
ACADEMIC EDITOR: Gabor Patonay, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,321 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: TI and TB. Analyzed the data: TI and NJ. Wrote the first draft of the manuscript: TI. Contributed to the writing of the manuscript: TI, NJ, BN, AR, and EH. Agree with manuscript results and conclusions: NJ, BN, AR, and EH. Jointly developed the structure and arguments for the paper: BN and AR. Made critical revisions and approved final version: NJ, BN, AR, and EH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Pascoal A, Rodrigues S, Teixeira A, Feás X, Estevinho LM. Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem Toxicol. 2014;63:233–239. doi: 10.1016/j.fct.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Estevinho LM, Rodrigues S, Pereira AP, Feás X. Portuguese bee pollen: palynological study, nutritional and microbiological evaluation. Int J Food Sci Technol. 2012;47(2):429–435. [Google Scholar]
- 3.Nogueira C, Iglesias A, Feás X, Estevinho LM. Commercial bee pollen with different geographical origins: a comprehensive approach. Int J Mol Sci. 2012;13(9):11173–11187. doi: 10.3390/ijms130911173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feás X, Vázquez-Tato MP, Estevinho L, Seijas JA, Iglesias A. Organic bee pollen: botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules. 2012;17(7):8359–8377. doi: 10.3390/molecules17078359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iglesias A, Feás X, Rodrigues S, et al. Comprehensive study of honey with protected denomination of origin and contribution to the enhancement of legal specifications. Molecules. 2012;17(7):8561–8577. doi: 10.3390/molecules17078561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva JC, Rodrigues S, Feás X, Estevinho LM. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem Toxicol. 2012;50(5):1790–1795. doi: 10.1016/j.fct.2012.02.097. [DOI] [PubMed] [Google Scholar]
- 7.Morais M, Moreira L, Feás X, Estevinho LM. Honeybee-collected pollen from five Portuguese Natural Parks: palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem Toxicol. 2011;49(5):1096–1101. doi: 10.1016/j.fct.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Feás X, Iglesias A, Rodrigues S, Estevinho LM. Effect of Erica sp. honey against microorganisms of clinical importance: study of the factors underlying this biological activity. Molecules. 2013;18(4):4233–4246. doi: 10.3390/molecules18044233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estevinho LM, Feás X, Seijas JA, Pilar Vázquez-Tato M. Organic honey from Trás-Os-Montes region (Portugal): chemical, palynological, microbiological and bioactive compounds characterization. Food Chem Toxicol. 2012;50(2):258–264. doi: 10.1016/j.fct.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Feás Z, Estevinho ML. A survey of the in vitro antifungal activity of heather (Erica Sp.) organic honey. J Med Food. 2011;14(10):1284–1288. doi: 10.1089/jmf.2010.0211. [DOI] [PubMed] [Google Scholar]
- 11.Estevinho ML, Afonso SE, Feás X. Antifungal effect of lavender honey against Candida albicans, Candida krusei and Cryptococcus neoformans. J Food Sci Technol. 2011;48(5):640–643. doi: 10.1007/s13197-011-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes T, Feás X, Iglesias A, Estevinho LM. Study of organic honey from the Northeast of Portugal. Molecules. 2011;16(7):5374–5386. doi: 10.3390/molecules16075374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feás X, Pires J, Iglesias A, Estevinho ML. Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physicochemical data. Food Chem Toxicol. 2010;48(12):3462–3470. doi: 10.1016/j.fct.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Feás X, Pires J, Estevinho ML, Iglesias A, De Araujo JPP. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. Int J Food Sci Technol. 2010;45(6):1255–1262. [Google Scholar]
- 15.Oksana S, Marian B, Mahendra R, Bo SH. Plant phenolic compounds for food, pharmaceutical and cosmetics production. J Med Plants Res. 2012;6(13):2526–2539. [Google Scholar]
- 16.Câmara C, Urrea C, Schlegel V. Pinto beans (Phaseolus vulgaris L.) as a functional food: implications on human health. Agriculture. 2013;3(1):90–111. [Google Scholar]
- 17.Campone L, Piccinelli AL, Pagano I, et al. Determination of phenolic compounds in honey using dispersive liquid-liquid microextraction. J Chromatogr A. 2014;1334:9–15. doi: 10.1016/j.chroma.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Jasicka-Misiak I, Poliwoda A, Deren M, Kafarski P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012;131(4):1149–1156. [Google Scholar]
- 19.Dimitrova B, Gevrenova R, Anklam E. Analysis of phenolic acids in honeys of different floral origin by solid-pase extraction and high-performance liquid chromatography. Phytochem Anal. 2007;18(1):24–32. doi: 10.1002/pca.948. [DOI] [PubMed] [Google Scholar]
- 20.Tomas-Barberan FA, Ferreres F, Garcia-Viguera C, Tomas-Lorente F. Flavonoids in honey of different geographical origin. Z Lebensm Unters Forsch. 1993;196:38–44. [Google Scholar]
- 21.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truchado P, Ferreres F, Bortolotti L, Sabatini AG, Tomas-Barberan FA. Nectar flavonol rhamnosides are floral markers of Acacia (Robinia pseudacacia) honey. J Agric Food Chem. 2008;56(19):8815–8824. doi: 10.1021/jf801625t. [DOI] [PubMed] [Google Scholar]
- 24.Escuredo O, Silva LR, Valentão P, Seijo MC, Andrade PB. Assessing Rubus honey value: pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012;130(3):671–678. [Google Scholar]
- 25.Martos I, Cossentini M, Ferreres F, Tomas-Barberan FA. Flavonoid composition of Tunisian honeys and propolis. J Agric Food Chem. 1997;45(8):2824–2829. [Google Scholar]
- 26.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73(9):R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 27.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 28.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100:114–117. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Pyrzynska K, Biesaga M. Analysis of phenolic acids and flavonoids in honey. Trends Analyt Chem. 2009;28(7):893–902. [Google Scholar]
- 30.Amiot MJ, Aubert S, Gonnet M, Tacchini M, Les MT. Les composés phéno-liques des miels: étude préliminaire sur l’identification et la quantification par familles. Apidologie. 1989;20(2):115–125. [Google Scholar]
- 31.Michalkiewicz A, Biesaga M, Pyrzynska K. Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J Chromatogr A. 2008;1187(1–2):18–24. doi: 10.1016/j.chroma.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Cavazza A, Corradini C, Musci M, Salvadeo P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J Sci Food Agric. 2013;93:1169–1175. doi: 10.1002/jsfa.5869. [DOI] [PubMed] [Google Scholar]
- 33.Proestos C, Komaitis M. Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC-MS after silylation. Foods. 2013;2(1):90–99. doi: 10.3390/foods2010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogdanov S. Harmonised Methods of the International Honey Commission. 2009. [Accessed October 5, 2015]. Available at: http://www.ihc-platform.net/ihcmethods2009.pdf.
- 35.Azeredo LC, Azeredo MAA, de Souza SR, Dutra VML. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003;80:249–254. [Google Scholar]
- 36.Chan CW, Deadman BJ, Manley-harris M, Wilkins AL, Alber DG, Harry E. Analysis of the flavonoid component of bioactive New Zealand manuka (Leptospermum scoparium) honey and the isolation, characterisation and synthesis of an unusual pyrrole. Food Chem. 2013;141(3):1772–1781. doi: 10.1016/j.foodchem.2013.04.092. [DOI] [PubMed] [Google Scholar]
- 37.Lachman J, Hejtmankova A, Sykora J, Karban J, Orsak M, Rygerova B. Contents of major phenolic and flavonoid antioxidants in selected Czech honey. Czech J Food Sci. 2010;28(5):412–426. [Google Scholar]
- 38.Vanderghem C, Jacquet N, Richel A. Can lignin wastes originating from cellulosic ethanol biorefineries act as radical scavenging agents? Aust J Chem. 2014;67:1693–1699. [Google Scholar]
- 39.Ball DW. The chemical composition of honey. J Chem Educ. 2007;84(10):1643–1646. [Google Scholar]
- 40.Desmouli A. Le miel: origine et composition. Actual Pharm. 2013;531:18–21. [Google Scholar]
- 41.Crane E. Honey: A Comprehensive Survey. London: Heinemann; 1975. [Google Scholar]
- 42.Chua LS, Lee JY, Chan GF. Honey protein extraction and determination by mass spectrometry. Anal Bioanal Chem. 2013;405:3063–3074. doi: 10.1007/s00216-012-6630-2. [DOI] [PubMed] [Google Scholar]
- 43.Caccamese S, Azzolina R. Separation of cis and trans isomers of naturally occuring hydroxycinnamic acids by high-pressure liquid chromatography. Chromatographia. 1979;12(8):545–547. [Google Scholar]
- 44.Vončina DB, Razboršek MI, Simonič M. High-performance liquid chromatographic determination of selected phenolic acids in wine. Nova Biotechnol. 2009;9(2):113–118. [Google Scholar]
- 45.Kato Y, Umeda N, Maeda A, Matsumoto D, Kitamoto N, Kikuzaki H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J Agric Food Chem. 2012;60:3418–3423. doi: 10.1021/jf300068w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. HPLC-DAD method.
Supplementary table 2. Retention time and m/z ratio of phenolic compounds in LC-UV-MS.
Supplementary table 3. LC-UV-MS method.
Supplementary figure 1. Acacia honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: caffeic acid, 2: p-coumaric acid, 3: chrysin).
Supplementary figure 2. Chestnut honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: p-coumaric acid, 2: chrysin, 3: pinocembrin).
Supplementary figure 3. Eucalyptus honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: caffeic acid, 2: quercetin, 3: kaempferol).
Supplementary figure 4. Thyme honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: p-coumaric acid, 2: naringenin, 3: chrysin, 4: pinocembrin).
Supplementary figure 5. Wild carrot honey extract chromatogram at 320 nm obtained with the LC-UV-MS method (1: caffeic acid, 2: p-coumaric acid, 3: chrysin).