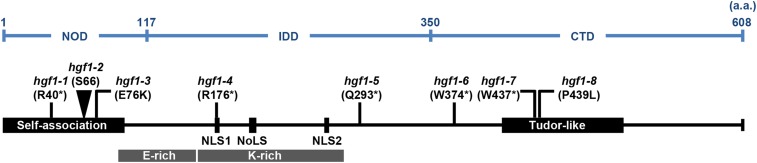
Figure 2.
Domain organization of Arabidopsis coilin and the positions of hgf loss-of-function mutations. Arabidopsis coilin consists of 608 amino acids. The N-terminal self-association domain and C-terminal Tudor-like domain are the most highly conserved regions of coilin proteins. Analysis of the secondary structure of Arabidopsis coilin predicted three domains: the N-terminal globular domain (NOD), the internal disordered domain (IDD), and the C-terminal domain (CTD) (Makarov et al., 2013). Also shown are two nuclear localization (NLS) signals and one cryptic nucleolar localization signal (NoLS) as well as E-rich and K-rich domains (Makarov et al., 2013). The eight hgf mutations retrieved in our forward genetic screen are indicated. Some alleles (hfg1-1, hgf1-3, hgf1-6, and hgf1-8) were obtained more than once. The corresponding nucleotide changes are shown in Figure S2.