Figure 1.
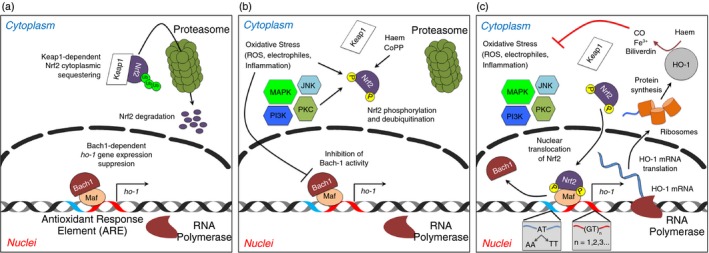
Hmox1 gene expression and its regulation. (a) In resting state, Nrf2 remains as bound to Keap1 in the cytoplasm. Nrf2 is constantly being ubiquitinated and targeted for proteasomal degradation. During this process, Bach‐1 heterodimerizes with small Mafs proteins at Antioxidant Response Elements (ARE) in the Hmox1 gene promoter site. (b) During cell stress caused either by pathogen‐associated molecular pattern (PAMPs) and/or pro‐oxidant molecules, Nrf2 is released from Keap1, increasing its stability and reducing its proteasome‐dependent degradation. Different kinases access to phosphorylate Nrf2 and activate its translocation to the nucleus. The binding of Bach‐1 to small Mafs is compromised by the same pro‐inflammatory and pro‐oxidants signals. (c) Phosphorylated Nrf2 migrates to the nucleus and displaces Bach‐1. Heterodimers Nrf2/Maf induce the activation of the Hmox1 gene promoter site and the recruitment of the RNA polymerase. The Hmox1 gene transcription begins. Depending on the (GT)n (n = number of repetitions) and the 413A > T (AT ‐> AA; AT ‐> TT) polymorphisms present in the promoter site, the amount of mRNA haem oxygenase 1 can vary.
