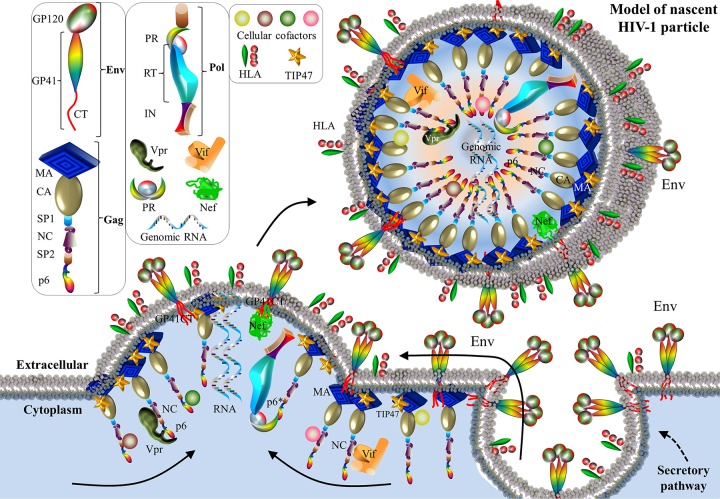
FIG 9.
Schematic model of HIV-1 protein interactions during viral budding. Env trimers are exported to the extracellular membrane through a secretory pathway (617). Gag and GagPol are targeted to glycolipid-enriched membrane lipid rafts, where cholesterol, sphingolipids, and glycosylphosphatidylinositol-linked proteins are abundant (618). Several HIV protein interactions have been observed. The first interaction is the matrixGag-GP41Env interaction. Mediated by the cellular cofactor TIP47, matrixGag interacts with GP41Env for Env packaging into HIV particles (297). Another is the Vpr-Gag interaction. Vpr is incorporated into nascent virions through the binding of Vpr to NCGag and p6Gag (315, 316, 324). A third interaction is the Vpx-p6Gag interaction. HIV-2 Vpx binds to p6Gag for Vpx packaging (333). A fourth interaction is the NCGag-Vif interaction. NCGag interacts with Vif for the packaging of Vif (300–303). A fifth interaction is the Env-Nef interaction. Nef enhances the packaging of Env (339, 341). On the viral membrane, ∼50 to 63 HLA-II complexes are incorporated per virion (619). As a capsid-encoding virus (620), HIV is featured by its fullerene cone harboring ∼250 hexamers and exactly 12 pentamers of HIV-1 capsid (285). Protein shapes do not indicate exact protein structures, nor are the protein sizes to scale.