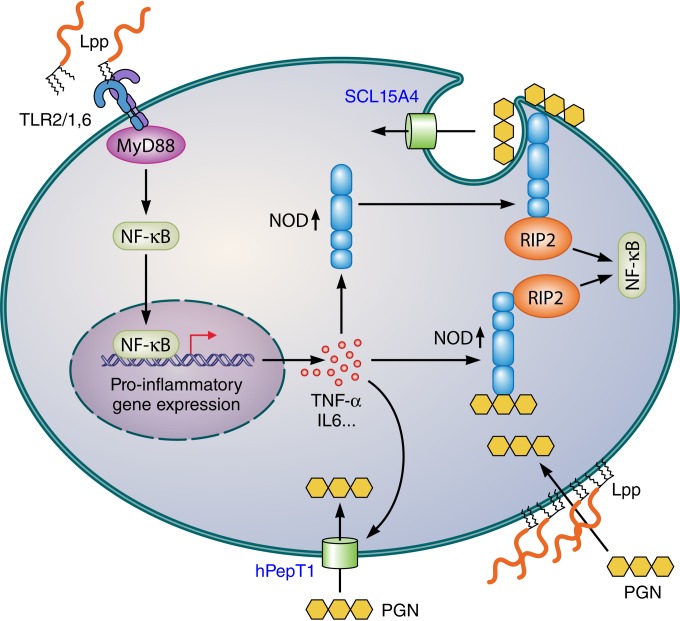
FIG 4.
Possible mechanisms for the synergistic immune stimulation of TLR2 and NOD2 ligands. Stimulation with di- and triacylated Lpp triggers the TLR2-MyD88-dependent signaling pathway, resulting in NF-κB activation and induction of proinflammatory cytokines. The latter, particularly TNF-α, upregulate NOD expression as well as oligopeptide transporters, e.g., hPepT1 and SCL15A4, that also transport NOD agonists. PGN is also taken up in an endocytosis-like process, from where it can be translocated into cytoplasm by the endosomal transporter SCL15A4. How NODs are recruited to the plasma membrane is unknown. Lpp not only act as TLR2 agonists but are also able to cause membrane scrambling; whether this effect contributes to signaling or to facilitating PGN uptake is unknown. Both TLR2 and NOD activation lead finally to NF-κB activation, but the downstream adaptor molecules, RIP2 and MyD88, are different, thus excluding competition for a common adaptor protein and allowing synergistic NF-κB activation. PGN, peptidoglycan; Lpp, lipoproteins/lipopeptides; MyD88, myeloid differentiation primary response protein 88; NF-κB, nuclear factor kappa-light-chain enhancer; NOD, nucleotide binding oligomerization domain-containing protein; RIP2, receptor-interacting serine/threonine protein kinase 2.