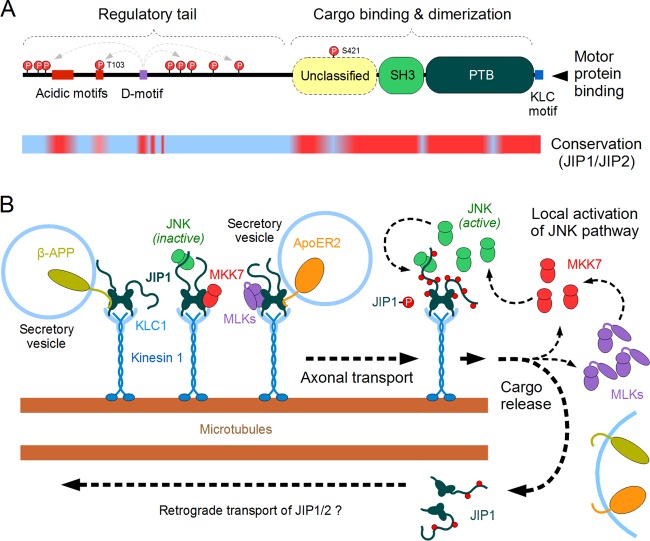
FIG 2.
Structure and function of JIP1 acting within the JNK pathway. (A) The domain architecture of the JIP1 protein. The N-terminal regulatory “tail” is largely disordered, while the C-terminal half of JIP1 contains three folded domains as well as a kinesin light chain (KLC)-binding linear motif. The precise function of the intrinsically disordered N terminus (with its conserved acidic motifs) is unknown, yet it is highly phosphorylated by JNK in a D-motif-dependent manner. Currently, only two target sites (T103 and S421) are known to have a role in JNK-dependent physiological regulation of JIP1. This model was built by combining domain signature searches (PFAM), folding tendency predictors (IUPRED), and conservation analyses (multiple alignments among vertebrate proteins) as well as curated data from the literature. The lower line shows the results of conservation analyses (red, highly conserved sequence; blue, nonconserved sequence), when sequences of vertebrate JIP1 and the closely related JIP2 proteins are aligned with each other. Structural domains and key motifs are preserved in both proteins (including the JNK-binding D-motif), while most regulatory phosphorylation sites differ between JIP1 and JIP2. (B) A model of JIP1 actions on the microtubule-dependent transport processes in neurons. The JIP1/2 dimers (turquoise) are capable of transporting a diverse set of membrane-associated proteins (e.g., β-APP, APoE2-R) as well as certain MAP2Ks (MKK7 [red]) and inactive MAP3Ks (MLK3, DLK [magenta]). These complexes are moved along the microtubule filaments with the help of kinesin 1-kinesin light chain 1 motors (blue). At the end of their journey, the transport complexes are uncoupled by a JNK-dependent phosphorylation of JIP1. Since this step also results in the release of upstream components and activators belonging to the JNK pathway, it leads to a positive feedback loop and helps to maintain subcellular compartments with high local JNK activity. The JIP1/2 proteins uncoupled from their cargo are also transported in a reverse direction (likely through a dynein-driven process), although the structural details of the latter complex are poorly known.