FIG 6.
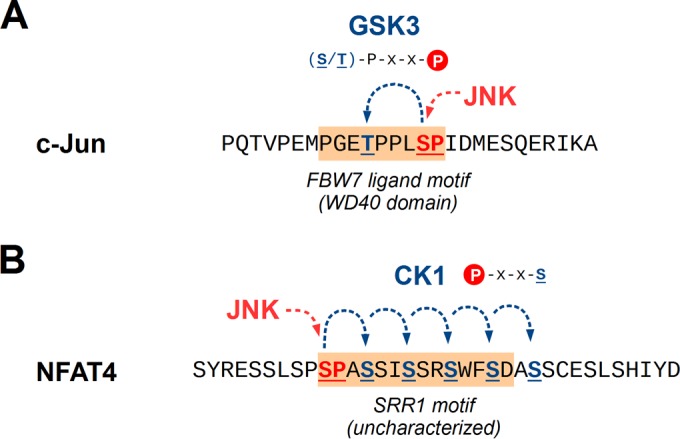
Cooperation between JNK and other kinases in substrate phosphorylation. (A) Phosphorylation by JNK can serve as a priming site for GSK3 enzymes in several substrates. The latter targets the site 4 amino acids upstream of the priming site, with a preference for proteins where the upstream Ser/Thr is also followed by a small amino acid, such as Pro. The double-phosphorylated region can often act as a phosphodegron, as in the case of c-Jun, where the motif is subsequently recognized by the cullin/F-box ubiquitin ligase FBW7. (B) Sites phosphorylated by JNK can also be recognized by casein kinase 1 (CK1). These enzymes phosphorylate Ser/Thr residues 3 amino acids downstream of the original phosphorylation site, with few sequence constraints. Like most other kinases reliant on substrate priming, CK1 can also recognize sites phosphorylated by itself. In the case of NFAT4, this leads to a chain of phosphorylation events initiated by JNK. Multisite phosphorylation of this so-called SRR1 (serine-rich region 1) motif then leads to cytoplasmic anchoring of NFAT4, although its precise binding partners are unknown.
