FIG 7.
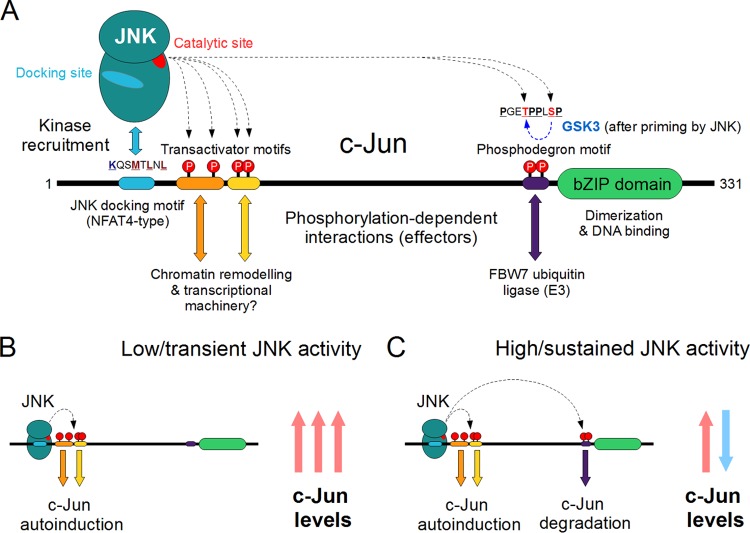
JNK-dependent phosphorylation of c-Jun elicits diverse effects. (A) The three Jun proteins (c-Jun, JunB, and JunD) were the first JNK substrates to be described and are still perhaps the best-known targets of the JNK pathways. The c-Jun protein can be phosphorylated at several sites by JNK; the most important regulatory sites are located in three different linear motifs. Phosphorylation of the two transactivator motifs located directly next to the docking motif can elicit transcriptional activation, probably due to phosphorylation-dependent recruitment of unknown effector proteins. This results in transcriptional activation of genes containing a Jun (AP-1)-binding element on their promoters, including c-Jun itself. On the other hand, phosphorylation of a much more distant phosphodegron motif in c-Jun, either by JNK alone or by cooperating with GSK3, represents an opposing regulatory mechanism. The latter provides a way for negative regulation of c-Jun levels by JNK, the mechanism for which is interestingly absent in the oncogenic v-Jun. (B) Under low or transient JNK activity, the c-Jun phospho-sites directly adjacent to the D-motif are the first to be phosphorylated, due to their favorable stereochemistry and strong coupling with the docking site. This results in a sharp rise of c-Jun mRNA and protein levels, due to autoinduction of the c-Jun gene. (C) If JNK activity is high and/or sustained over several hours, efficient phosphorylation of the distant C-terminal sites may also occur. The result will be the well-known attenuation of c-Jun levels due to ubiquitinylation and proteasome-dependent degradation of phospho–AP-1 complexes.