FIG 8.
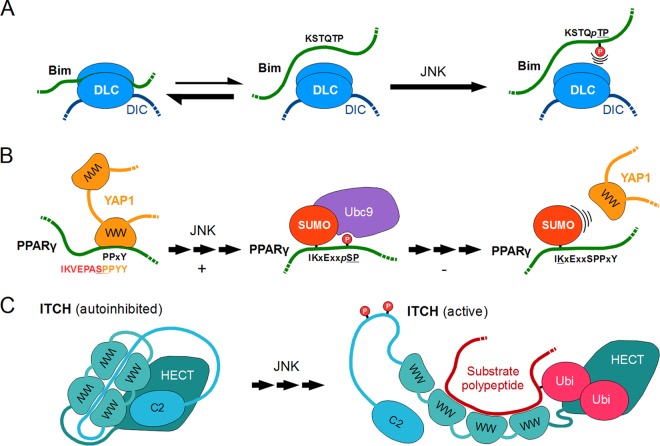
Negative phospho-switches and autoinhibitory switches driven by JNK. (A) A simple negative switch, illustrated by Bim. Phosphorylation of a dynein light chain (DLC)-binding motif in the BH3-only apoptosis regulator protein Bim impedes its binding to DLC-DIC (dynein intermediate chain) complexes. This shifts the equilibrium toward free Bim molecules, eventually completely releasing them into the cytoplasm. (B) A complex negative switch occurring on PPARγ. The disordered N terminus of the peroxisome proliferator-activated receptor γ contains a WW domain ligand and an overlapping PDSM (phosphorylation-dependent SUMOylation motif). Phosphorylation by JNK can therefore elicit SUMOylation of PPARγ, making its association with WW domain-containing coactivators (such as YAP1) sterically impossible. Ubc9 is a SUMO-conjugating enzyme. (C) The autoinhibitory switch of the ITCH ubiquitin ligase. As typical for the NEDD4 family of E3 ubiquitin ligases, ITCH is subject to autoinhibition. According to studies, the autoinhibitory interface of ITCH is complex, and the C2 domain, linkers, and its WW domains all contribute to maintenance of the “closed” conformation. JNK-dependent phosphorylation of certain residues located in the linker region interfere with the autoinhibitory interactions. This “opens up” the catalytic HECT domain of the ITCH enzyme, allowing the E3 ligase to recruit and ubiquitinylate its substrates.