Fig. 7.
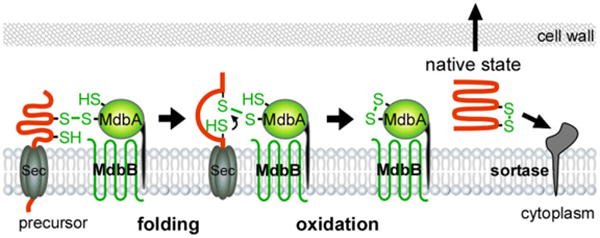
A model of MdbA-mediated posttranslocational protein folding. The transmembrane thiol-disulfide oxidoreductase MbdA enzyme forms a mixed disulfide bond with nascent precursors emerging from the Sec translocon, allowing the precursors to fold into a near-native state before MdbA-driven catalysis of disulfide bond formation, resulting in fully folded proteins for subsequent steps. An unidentified membrane-bound oxidoreductase termed MdbB is proposed to reoxidize MdbA.
