Abstract
Stereotactic radiotherapy (SRT) is a useful treatment for malignant ling tumors. However, SRT is associated with complications such as high local recurrence rate and radiation-induced lung injury. Herein, we report a case of combined aortic resection for after SRT. An 82-year-old man underwent SRT for the metastatic lung carcinoma of rectal cancer at left lower lobe. Three years later, chest computed tomography showed local recurrence at the site of radiotherapy, with suspected invasion of the descending aorta. Thoracotomy was performed after metastatic lung carcinoma interpolation of a stent graft in the descending aorta. Because the tumor firmly adhered to the aorta, left lower lung lobe and aortic wall resection was performed. Pathological findings revealed fibrous hypertrophy and adhesion between the visceral pleura and aorta. As shown in our case, combined aortic resection and stent graft insertion is an effective minimally invasive and safe treatment for SRT-induced tissue damage.
Keywords: stent graft, stereotactic radiotherapy, combined aortic resection, local recurrence after radiotherapy, chronic radiation fibrosis
Introduction
Stereotactic radiotherapy (SRT) is one of the most useful treatments for lung cancer patients who are elderly or for whom surgery is considered very risky. However, SRT can cause complications including a higher rate of local tumor recurrence compared to surgery,1,2) and advanced fibrosis in normal lung parenchyma and radiation-induced injury.3,4)
We report a case of metastatic lung carcinoma that firmly adhered to the descending aorta after SRT and was treated with combined aortic resection and stent graft insertion.
Case Report
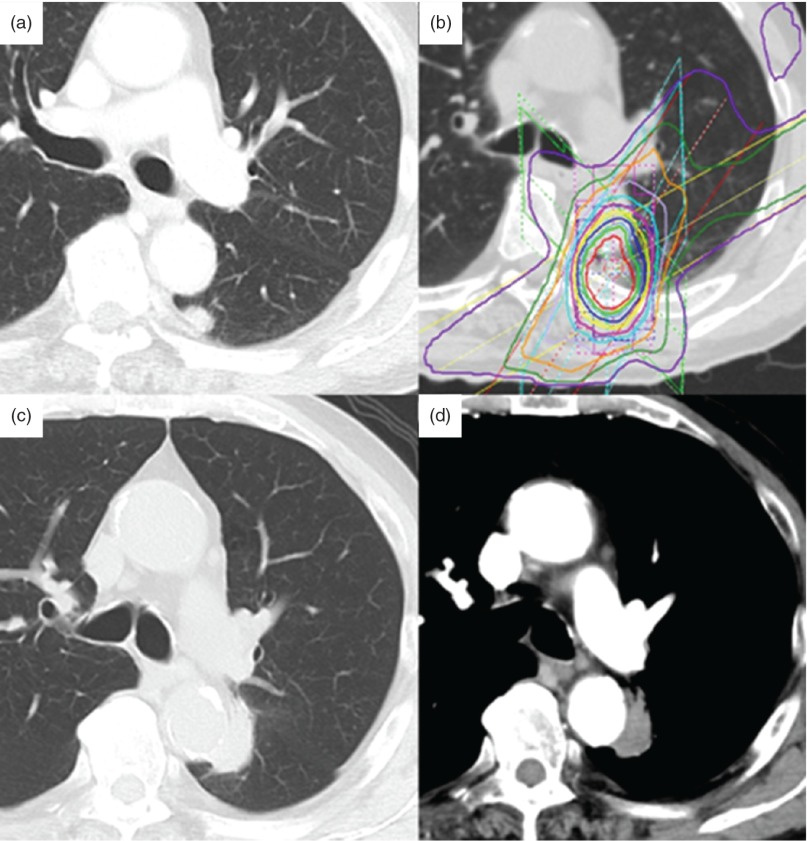
An 82-year-old man with no history of smoking underwent resection of stage I rectal cancer 15 years previously. Twelve years later, an abnormal shadow was detected in segment 6 of the left lung chest computed tomography (CT). Positron emission tomography-CT (PET-CT) revealed soft tissue opacity and an irregular nodule 7 mm in size, with a standardized uptake value of 7.3 (Fig. 1a). The patient was considered a candidate for resection of the suspected metastatic lung carcinoma. However, since the patient strong desired radiotherapy, he underwent SRT of 48 Gy/4f (Fig. 1b). Following SRT, the nodule disappeared. However, 3 years after SRT, a 10 × 5-mm nodule was detected in segment 6 of the left lung on chest CT. The nodule was diagnosed as a local cancer recurrence probably due to SRT, and was suspected to have invaded the aorta because of its border was unclear and because it was in contact with the descending aorta (Figs. 1c, 1d). We decided on surgical resection because the abnormal lesion was confined to the lung. Since this elderly patient had advanced atherosclerosis, surgery using cardiopulmonary bypass was considered very risk. Therefore, we elected to perform a stent graft combination procedure. The operation was performed under general anesthesia with the patient in the supine position. We approached the aorta from the right femoral artery. A thoracic endovascular stent graft (COOK Zenith TX2 endovascular graft: 34 × 152 mm; COOK JAPAN Inc, Tokyo, Japan) was inserted into the thoracic aorta (Fig. 2). After endovascular treatment, we performed thoracotomy by posterolateral incision in the right decubitus position. The region between the tumor and the aorta could not be separated, and so we performed combined resection of the aortic wall (Fig. 3a). It was not used the aortic clamp and cardiopulmonary bypass. Only a small amount of bleeding occurred during the operation, so transfusion was not needed. Left lower lobectomy and partial resection of upper lobe were performed because of invasion of the inter lobe. The aortic wall defect was reinforced with a vascular graft patch (Triplex 30 mm straight type; TERMO Inc, Tokyo, Japan) with 4-0PDS (Ethicon Inc, Somervill, NJ) running suture (Fig. 3b).
Fig. 1.
Chest CT findings. (a) Chest CT 3 years ago shows the lesion of nodule in left lower lobe. (b) Stereotactic radiotherapy treatment planning image. (c, d) Three years after SRT, an abnormal shadow appeared. PET-CT revealed soft tissue opacity and an irregular nodule 7 mm in size, leading to a diagnosis of local recurrence of lung cancer. CT: computed tomography; SRT: stereotactic radiotherapy; PET-CT: positron emission tomography-CT
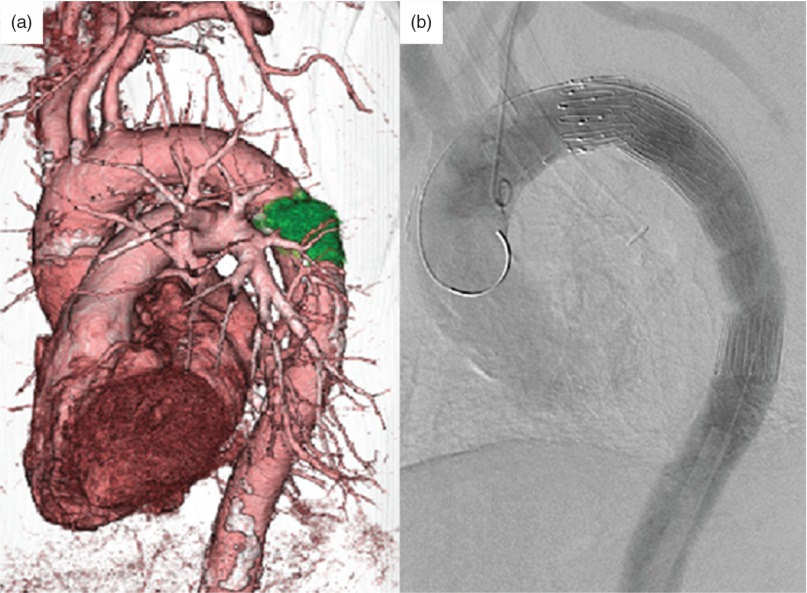
Fig. 2.
Stent graft interpolated. The stent graft was positioned on the aorta and covering the surgical margin of the tumor. The tumor’s location was confirmed using 3D-CT. CT: computed tomography
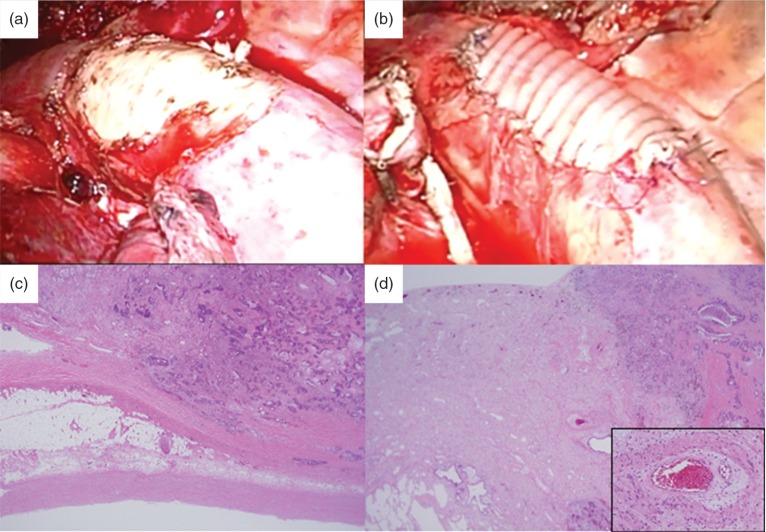
Fig. 3.
Surgical and pathological findings. (a) We performed combined aortic resection. Only a small amount of bleeding resulted from insertion of the stent graft. (b) The aortic wall defect was reinforced with a vascular graft patch. (c) Hematoxylin-Eosin stain shows fibrous hypertrophy and adhesions between the visceral pleura and the aorta. (d) An interstitial pneumonitis with cicatricial perivascular fibrosis can be seen. The small muscular arteries exhibit prominent myointimal proliferation and intramural hyalinization. There are pathological changes indicating chronic radiation fibrosis.
The postoperative course was uneventful, and the patient was discharged 10 days after the operation. Pathological findings revealed that the tumor was a metastatic lung carcinoma secondary to rectal cancer. The cancer had invaded the upper lobe of the lung but not the aorta or pulmonary artery. There were thick fibrous adhesions on the aortic wall and in the visceral pleura. This fibrosis also involved all segments of the lung, most notably the lung parenchyma and vasculature. We also observed architectural collapse and irreversible scarring of the lung parenchyma as well as prominent myointimal proliferation and intramural hyalinization of the small muscular arteries (Figs. 3c, 3d).
These findings suggested that SRT had caused the tight cicatricial adhesions. Eight months after surgery there was no evidence of local recurrence or distant metastasis.
Discussion
Stereotactic radiotherapy (SRT) is the treatment of choice for peripheral lung malignant tumors in inoperable patients. However, the overall local recurrence rate is 10.2%, which is significantly higher than the 2.6% recurrence rate following surgery.1) In addition, SRT’s high-dose radiation causes lung injury that leads to advanced local inflammation.
In the early phase (six months or less) following radiation-induced injury, numerous intra-alveolar macrophages and red blood cells can be seen that are associated with acute diffuse alveolar damage. The radiological image reveals grand grass attenuation (GGA).5,6) In the later phase (after two years), these changes become localized to the area of the radiation port, with fibrosis affecting all compartments of the lung, most notably the lung parenchyma and vasculature.7)
The radiological image reveals bronchiectasis similar to but less extensive than conventional radiation fibrosis, scar-like fibrosis (linear opacity in the region of the original tumor) and mass-like fibrosis.5,6) Therefore, in this case, advanced adhesions formed without tumor invasion. This should be taken into consideration when performing salvage surgery.
Marulli et al. in 2008, reported a case in which stent graft was applied to aortic resection for the first time.8) Later, similar procedures were reported in ten cases,8–12) six of them primary lung cancer cases and four of them metastatic lung tumors. The procedures consisted of two pneumonectomies, six lobectomies and two partial resections. For four of the cases, the excision site was the aortic arch, and for six of the cases the excision site was the descending aorta. In four cases, the full thickness of the aortic wall was excised and it was possible to safely complete the resection. After resection of our patient, we carried out patch repair by vascular graft appropriate for the given the aorta strength.
In addition, we have done the implantation of intra-aortic stent procedure and lung resection on the same day. The fact that this technique is possible, when the unpredictable bleeding, invasion, or adhesion were found, it is possible to cope with these situations by inserting the stent graft during surgery. In previous reports, none was carried out them at the same time. We believe that this technique lead to future development.
Conclusion
Tight adhesions can form between adjacent organs following SRT. We showed that it is possible to safely perform minimally invasive combined aortic resection by insertion of a stent graft.
Disclosure of Conflict of Interest
None.
References
- 1).Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014; 147: 1183-91; discussion 1191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015; 99: 1122-9. [DOI] [PubMed] [Google Scholar]
- 3).Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)–can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012; 102: 335-42. [DOI] [PubMed] [Google Scholar]
- 4).Kawase T, Takeda A, Kunieda E, et al. Extrapulmonary soft-tissue fibrosis resulting from hypofractionated stereotactic body radiotherapy for pulmonary nodular lesions. Int J Radiat Oncol Biol Phys 2009; 74: 349-54. [DOI] [PubMed] [Google Scholar]
- 5).Huang K, Palma DA, IASLC Advanced Radiation Technology Committee. Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. J Thorac Oncol 2015; 10: 412-9. [DOI] [PubMed] [Google Scholar]
- 6).Trovo M, Linda A, El Naqa I, et al. Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT). Lung Cancer 2010; 69: 77-85. [DOI] [PubMed] [Google Scholar]
- 7).O'Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol 2003; 13: 274-89. [DOI] [PubMed] [Google Scholar]
- 8).Marulli G, Lepidi S, Frigatti P, et al. Thoracic aorta endograft as an adjunct to resection of a locally invasive tumor: a new indication to endograft. J Vasc Surg 2008; 47: 868-70. [DOI] [PubMed] [Google Scholar]
- 9).Roche-Nagle G, de Perrot M, Waddell TK, et al. Neoadjuvant aortic endografting. Ann Vasc Surg 2009; 23: 787.e1-5. [DOI] [PubMed] [Google Scholar]
- 10).Berna P, Bagan P, De Dominicis F, et al. Aortic endostent followed by extended pneumonectomy for T4 lung cancer. Ann Thorac Surg 2011; 91: 591-3. [DOI] [PubMed] [Google Scholar]
- 11).Nagata T, Nakamura Y, Yamamoto H, et al. A fenestrated stent graft for surgical resection of lung cancer invading the aortic arch. J Thorac Cardiovasc Surg 2013; 146: 238-9. [DOI] [PubMed] [Google Scholar]
- 12).Collaud S, Waddell TK, Yasufuku K, et al. Thoracic aortic endografting facilitates the resection of tumors infiltrating the aorta. J Thorac Cardiovasc Surg 2014; 147: 1178-82; discussion 1182. [DOI] [PubMed] [Google Scholar]