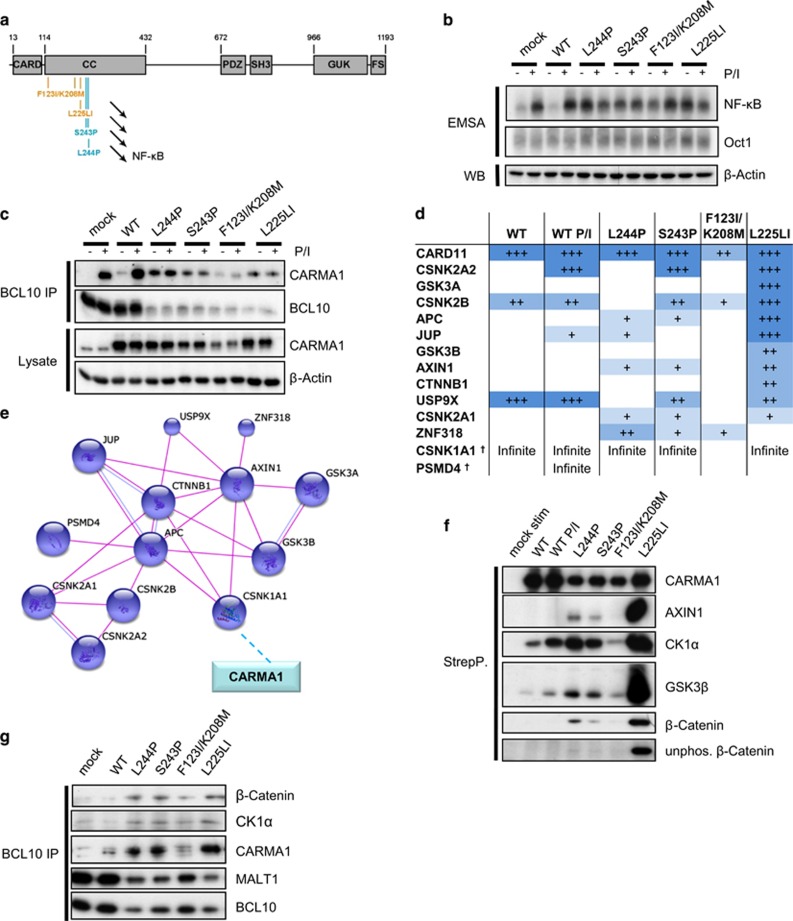
Figure 1.
Proteomic analysis defines a functional interaction of oncogenic CARMA1 with the β-catenin destruction complex. (a) Schematic depiction of CARMA1 domains and generated ABC (blue) or GCB (orange) DLBCL-derived mutations of the coiled-coil (CC) domain. CARD: caspase recruitment domain; SH3: SRC homology 3; GUK: guanylate kinase; FS: Flag-Strep-Tag. (b) Oncogenic CARMA1 promotes constitutive NF-κB activity. NF-κB DNA binding was assessed in EMSA of unstimulated or 60 min P/I stimulated transduced BJAB cells. Oct1 EMSA was performed for control. (c) Oncogenic CARMA1 is constitutively recruited to BCL10-MALT1. CBM complex formation was assessed in stimulated (15 min P/I) or unstimulated transduced BJAB cells and analyzed in western blot after BCL10 IP. (d) LC-MS/MS identifies CARMA1 interaction partners consisting of proteins surrounding the β-catenin destruction complex. Three independent Strep-precipitation experiments were conducted in CARMA1 WT, WT stimulated (30 min P/I) and four different oncogenic CARMA1 mutant-transduced BJAB. The ratio of mean peptide abundances of each sample related to the mock precipitation control was calculated and depicted with +=2–10, ++=10–50 and +++=>50. Infinite stands for incalculable values, because no peptides were detected in mock control. † marks proteins that were identified by one single peptide. (e) Interaction database analysis of identified CARMA1 interaction partners emerges a network of proteins surrounding the β-catenin destruction complex. Known and predicted protein interactions using string database (string-db.org) was calculated based on experimental data with a medium confidence score. Only significant interactions (P<0.05) were used for string analyses. Published CK1α-CARMA1 interaction was integrated manually with dashed line. (f) Oncogenic CARMA1 interacts with proteins of the β-catenin destruction complex. CARMA1 StrepTactin pull-down in transduced BJAB cells and associated proteins were analyzed in western blot. (g) Proteins of the β-catenin destruction complex are associated to the CBM complex. The interaction of CK1α and β-catenin to the CBM complex was assessed in transduced BJAB cells and analyzed in western blot after BCL10 IP.