Figure 4. Remote loading of liposomes with small molecules.
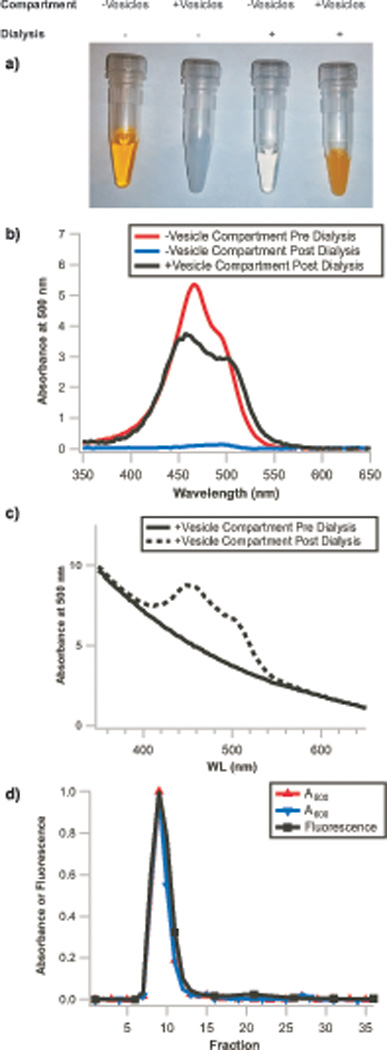
a) Images of vesicle-free and vesicle-containing solutions before and after dialysis. Selective accumulation of acridine orange occurs in the compartment containing vesicles as dye molecules are depleted from the vesicle-free compartment, demonstrating remote loading occurs. Similarly, b) The vesicle-free solution from the dialyzer, initially containing 100 µM acridine orange (red trace), is >95% depleted of dye (blue trace) after dialysis due to accumulation of dye within the vesicles (black trace, background corrected for scattering by subtraction of a vesicle blank). c) The non-background corrected post-dialysis vesicle sample (dashed line) exhibits the same A600 (only vesicle scattering contributes to absorbance at this wavelength) as the pre-dialysis vesicle sample (solid line), demonstrating that the vesicles are not diluted by this protocol. Absorbance signals are from NanoDrop measurements and are normalized to 10mm path length. d) Repurification of these vesicles results in superimposable normalized A500 (acridine orange absorbance), A600 (vesicle scattering), and acridine orange fluorescence traces (λex=500 nm, λem=530 nm), demonstrating that the dye has both crossed the dialysis membrane and accumulated within the vesicle lumen. Integration of fluorescence signal in the encapsulated dye fractions (fractions 6–16) and free dye fractions (fractions 17–27) indicates >95% encapsulation efficiency.
The methods for preparation of vesicles are described in our previous work1,21; the remote loading protocol (without the use of the dialyzer) was previously described elsewhere.14
