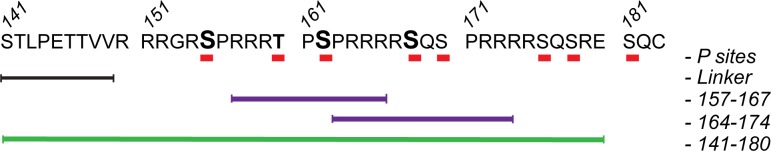
Fig 1. The C-terminal domain (CTD) of HBV core protein.
Cp183 is comprised of an assembly domain (residues 1–149) that includes a nine-residue linker (underlined by the black line, 141–149) [83], and a 34-residue CTD. The CTD has seven phosphorylatable serines and one phosphorylatable threonine (red lines). Phosphorylation of serines 155, 162, and 170 (large letters) appears to play a critical role in RNA-packaging [11]. Arginine-rich segments have been associated with nuclear localization signals [21–23,27]; as an example, two peptides that conform to a classical bipartite NLS sequences (purple lines) have been demonstrated to compete with known NLS sequences [8]. Cp183’s IBB segment has not been specifically identified but an IBB is ~40 amino acids including ~17 basic residues [28] and could thus incorporate most of the CTD as well as the linker region (green line); no other sequence in Cp183 has this wealth of basic residues.