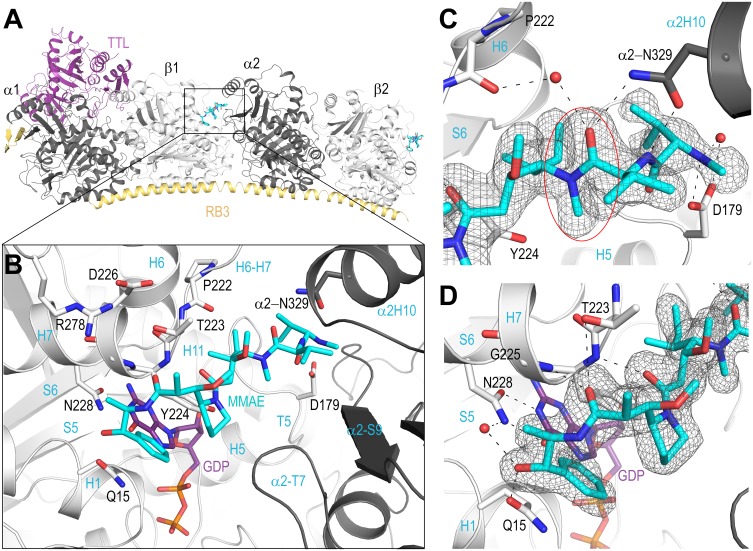
Fig 2. Location of the MMAE binding site in the T2R-TTL complex.
(A) The entire T2R-TTL complex is shown with TTL (purple) and RB3 (yelloworange), α (dark gray) and β tubulin (light gray) subunits. MMAE molecules (cyan) are bound to the complex at both the high affinity β1/α2 interface, and to the lower affinity β2 subunit alone, however density for the low affinity site is poorly resolved. (B) The high affinity MMAE binding site, colored as in panel A. The MMAE molecule makes the most extensive contacts with the H6-H7 loop on the β subunit, and the carboxy-terminal norephedrine is located directly above the GDP ligand. The amino-terminus of the molecule primarily interacts with the βT5 loop and αH10 on the adjacent subunit. (C) Specific interactions of the MMAE amino-terminal residues. Asp179 located on the T5 loop interacts with the positively charged N-methyl group and co-coordinates a crystallographic water. The side chain of Asn329 on αH10 forms a dual interaction with the amide nitrogen and carbonyl group of the MMAE valine residue. A crystallographic water molecule is also located between the MMAE valine carbonyl and the carbonyl of the Pro222 of the βH6-H7 loop. The trans-configuration observed at the Val-Dil amide bond of the bound MMAE is highlighted with a red ellipse. (D) Specific interactions of the MMAE carboxy-terminal residues. The carbonyl groups of both Dil and Dap form hydrogen bonds to the amide nitrogens of Tyr224 and Gly225, respectively. The carboxy-terminal norephedrine is positioned directly above the GDP ligand and the hydroxyl group forms interactions with the side chain of Gln15 and coordinates a crystallographic water molecule with Asn228. The SigmaA‐weighted mFo‐DFc omit map (grey mesh) in both the panels C and D is contoured at + 3.0σ.