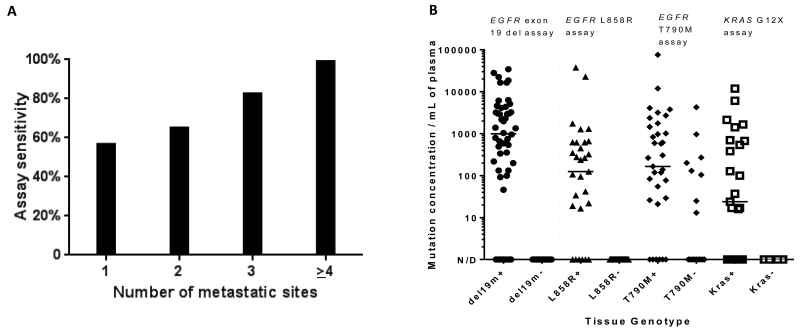
Figure 1.
(A) The sensitivity of plasma ddPCR for the detection of EGFR and KRAS mutations increases directly with the number of metastatic sites present in a given patient (p<0.001). (B) Dynamic range of plasma genotyping using a validated ddPCR-based assay. Wide dynamic range and the absence of false positives are noted for the detection of KRAS G12X and EGFR sensitizing mutations. A small number of false positives are seen with the EGFR T790M assay – potentially secondary to tumor heterogeneity with respect to acquired resistance mechanisms (n=174).