Abstract
Rationale
When ad libitum fed rats undergo cocaine place preference conditioning (CPP) but are switched to food restriction for testing, CPP becomes resistant to extinction and correlates with phosphorylation of AMPA receptor GluA1 at Ser845 in nucleus accumbens (NAc) core.
Objectives
This study tested whether food restriction increases persistence of morphine CPP and conditioned place aversions (CPA) induced by LiCl and naloxone-precipitated morphine withdrawal.
Materials and methods
Ad libitum fed rats were conditioned with morphine (6.0 mg/kg, i.p.), LiCl (50.0/75.0 mg/kg, i.p.), or naloxone (1.0 mg/kg, s.c.) 22 hours post-morphine (20.0 mg/kg, s.c.). Half the subjects were then switched to food restriction. Daily testing resumed three weeks later and brains were harvested when one diet group met extinction criterion. Western analyses probed for pSer845-GluA1, pERK1 and pERK2 in NAc.
Results
Food restriction increased persistence of morphine CPP and preference scores correlated with pSer845-GluA1 in NAc core and shell. LiCl CPA was curtailed by food restriction, yet pSer845-GluA1 and pERK2 were elevated in NAc core of food restricted rats. Food restriction increased persistence of naloxone CPA, elevated pSer845-GluA1 in NAc core and shell, and aversion scores were negatively correlated with pERK1 and pERK2 in NAc core.
Conclusions
These results suggest that food restriction prolongs responsiveness to environmental contexts paired with subjective effects of both morphine and morphine withdrawal. A mechanistic scheme, attributing these effects to upregulation of pSer845-GluA1, but subject to override by CPA-specific, pERK2-mediated extinction learning, is explored to accommodate opposite effects of food restriction on LiCl and naloxone CPA.
Keywords: Food restriction, nucleus accumbens, conditioned place preference, conditioned place aversion, GluA1, ERK
An early guiding hypothesis in drug abuse research, now well supported by several decades of research, is that drugs of abuse target and appropriate the neural substrates for appetitive motivation and reward. With a primary focus on mechanisms of energy homeostasis and ingestive behavior, CNS and behavioral effects of a wide array of abused drugs have been shown to be modulated by diet and patterns of ingestive behavior (Carroll et al. 1979; Cabeza de Vaca and Carr 1998; Wellman et al. 2001; Thanos et al. 2010; Puhl et al. 2011; Collins et al. 2015; Sedki et al. 2015). Most of this research has employed drug abuse models that involve acute drug challenge or acquisition/maintenance of self-administration behavior.
A hallmark characteristic of addiction, which undercuts the efforts of clinicians and abstinent drug users, is the craving and relapse induced by several identified triggers, one of which is environmental cues/contexts associated with previous drug use (Childress et al. 1988; O’Brien et al. 1992). The modulatory effects of diet, energy balance, and associated neural and endocrine factors on responsiveness to drug-associated cues and contexts have received little experimental attention. Using a conditioned place preference (CPP) paradigm, it has been shown that a cocaine CPP is more robust and resistant to extinction if subjects are switched from ad libitum feeding to chronic food restriction in the three week period that intervenes between conditioning and testing (Zheng et al. 2012). This observation is concordant with reports that food restriction enhances reinstatement of cocaine- (Carroll 1985) and heroin-seeking (D’Cunha et al. 2013) in self-administration protocols. The cocaine CPP studies have also revealed that the persistent CPP in food restricted subjects is not affected by the temporal relation between the most recent meal and testing, or meal-entrained levels of blood-borne metabolic hormones (Zheng et al. 2013), but correlates with phosphorylation of the glutamatergic AMPA receptor GluA1 subunit at Ser845 in nucleus accumbens core, and is blocked by accumbens core microinjection of Naspm, a selective antagonist of calcium-permeable AMPA receptors (Zheng et al. 2013; Zheng et al., 2015). This parallels findings relating to increased unconditioned drug reward in food restricted rats, though the accumbens subdivision implicated is the shell (Carr et al. 2010; Peng et al. 2014).
The purpose of the present study was to more fully characterize the effect of food restriction on conditioned responding to contexts. To test generality of the enhancing effect of food restriction on CPP expression, Experiment 1 compared morphine CPP expression in ad libitum fed and food restricted rats. Following observation of an enhancing effect similar to that seen in the cocaine CPP experiments, two additional experiments were conducted to determine whether the effect of food restriction is confined to reward-related behavior or extends to other forms of place conditioning and therefore might reflect alteration of a general function such as memory retrieval or extinction learning. Consequently, conditioned place aversions (CPA) induced by LiCl and naloxone-precipitated morphine withdrawal were examined. In all experiments, the association between diet, behavior and pSer845-GluA1, as well as pERK1/2, were examined in NAc core and shell.
Materials and methods
Subjects and food restriction
Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York University School of Medicine and were consistent with the Principles of Laboratory Care (NIH Publication no. 85–23). All subjects were mature male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 300–400 g (10–12 weeks old) at the start of each experiment. Rats were housed on a 12-hour light:dark photoperiod with lights on between 0600 and 1800 in a central animal facility. All subjects were singly housed in individual plastic cages with bedding and free access to water.
Ad libitum fed animals had free access to standard lab pellets (PicoLab Rodent Diet 20, LabDiet, St. Louis, MO). Food restricted animals followed a feeding regimen described previously (e.g., Cabeza de Vaca and Carr 1998), in which rats received 40% of ad libitum chow intake (10 g) daily, delivered to the home cage at 1700, until body weight was reduced by 20% (~2 weeks). Daily feeding was then titrated to maintain the new body weight for one week before resuming behavioral testing. Restricted feeding and body weight were maintained until the end of the experiment.
Place conditioning apparatus
Each conditioning apparatus consisted of two main cubicles (25.4 × 30.5 × 30.5 cm) separated by a center compartment (10.2 × 30.5 × 30.5 cm). Removable partitions separating the main cubicles from the center compartment had walls matching their respective cubicles and a small opening (12.5 × 15 cm) allowing movement between cubicles. Partitions without openings, but still matching the respective walls, were used to isolate subjects within a cubicle during conditioning. Automated data collection was accomplished through 24 infrared photo-beam detectors along the length of the test chamber (VersaMax System, Accuscan, Columbus, OH), and provided a record of horizontal activity and time spent in each compartment.
Habituation and pre-conditioning
All rats were habituated to transport and handling on at least five occasions. On the first experimental day, each rat was pre-exposed to the place conditioning apparatus. Pre-exposure involved placing the rat in the center compartment and allowing free movement for 20 min. Time spent in each compartment was recorded. Based on the absence of initial preference for either conditioning compartment, rats were randomly assigned to receive the experimental treatment prior to placement in one of the two compartments.
Experiment 1: Morphine Conditioning
At least 48 hours after pre-exposure, rats underwent a series of 8 consecutive daily conditioning sessions. Sessions were of 30 minutes duration, with partitions in place and animals confined to one side of the apparatus. On alternate days, rats were injected with morphine sulfate (6.0 mg/kg, i.p.) immediately prior to placement in the assigned compartment. On intervening days, rats were injected with saline-vehicle (1.0 ml/kg, i.p.) before confinement to the opposite compartment.
Experiment 2: LiCl conditioning
At least 48 hours after pre-exposure, rats underwent 12 conditioning sessions, of 20 minute duration, over consecutive days except as described below. On alternate days, rats were injected with LiCl (50.0 mg/kg, i.p.) immediately prior to placement in the assigned compartment. On intervening days, rats received saline-vehicle injections (1.0 ml/kg, i.p.) before confinement to the opposite side compartment. However, a CPA test conducted after the first eight days of conditioning did not reveal a statistically significant place aversion (t(23)=1.19, p=0.12). Consequently, rats were conditioned for four more days with 75.0 mg/kg (i.p.) LiCl alternating with saline control injections.
Experiment 3: Naloxone-precipitated morphine withdrawal conditioning
The conditioning phase of this experiment consisted of four cycles based on the method of Parker and colleagues (1998). On the first day of each cycle, rats were injected with saline-vehicle (1.0 ml/kg, s.c.) 5-min before placement in the assigned compartment for 30 min. One hour after removal from the compartment, rats were injected with morphine sulfate 20.0 mg/kg (s.c.) and remained in home cages. Twenty two hrs following morphine administration, rats were injected with naloxone (1.0 mg/kg, s.c.) 5-min before placement in the alternate compartment of the conditioning apparatus for 30 min. Cycles were separated by intervals of 48–72 hrs.
Testing
At least 48 hours after the last conditioning session, rats were tested for CPP or CPA expression. On test days, rats were placed in the center compartment and allowed to move freely throughout the apparatus for 20 min. Additional tests were conducted after a three-week interval, during which half the subjects had been switched from ad libitum feeding to a food restriction protocol that was maintained until the end of the experiment. Daily testing continued until one diet group displayed extinction of conditioned place behavior. Extinction was defined as three consecutive test sessions in which time spent on the experimental treatment-paired side of the conditioning apparatus did not differ from time spent on the saline-paired side, as determined by t-test. To ensure a conservative approach to establishing extinction, t-tests were each one-tailed with the α-level set at 0.05 (i.e., no correction for multiple tests); this maximized the likelihood of concluding a difference between time spent on the two sides, and thus no extinction. This was followed by at least one additional test session, enabling brain harvesting and biochemical assay immediately after.
Drugs
Morphine sulfate was obtained from the National Institute on Drug Abuse (NIDA), Research Triangle Institute, Research Triangle Park, NC, and dissolved in sterile 0.9% saline for subcutaneous injection. Naloxone hydrochloride dihydrate and LiCl (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile 0.9% saline for subcutaneous and intraperitoneal injection, respectively.
Biochemical measures
Immediately following the final expression test in each behavioral experiment, subjects were briefly exposed to CO2 and decapitated by guillotine. Brains were extracted and rapidly frozen in powdered dry ice. A series of 500-µm sections were cut using an IEC Minotome cryostat, and NAc core and shell and caudate-putamen were dissected using a combination of micropunch and microknife under an Olympus dissecting microscope. Tissue lysates were prepared as described previously (e.g., Zheng et al. 2013) and stored in sample buffer in aliquots at −80°C. The protein content was determined using the BCA reagent kit with bovine serum albumin as a standard (Pierce, Rockford, IL).
Western blotting
Proteins (6–10 µg/lane) were separated by electrophoresis on precast 4–12% sodium dodecyl sulfate polyacrylamide gels (Lonza, Rockland, ME). Precision Plus protein standard molecular weight markers (Bio-Rad) were loaded to estimate the size of the target proteins and to ensure complete transfer of proteins from gel to membrane. Proteins were electrophoretically transferred to Protran nitrocellulose membranes (Whatman, Mobile, AL) for 1.75 hours at a constant voltage of 100V.
Membranes were blocked for 60 minutes with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) with shaking at room temperature. Membranes were then probed overnight at 4°C using primary antibodies for target proteins or the protein loading control, α-tubulin. Primary antibodies were diluted in Odyssey Blocking Buffer with 0.2% Tween 20. After probing with primary antibodies and washing with TBST buffer (3 × 5 min), membranes were incubated for 1 hour at room temperature with IRDye 680RD Goat anti-Mouse IgG (H+L) and IRDye 800CW Goat anti-Rabbit IgG (H+L) (1:16,000; LI-COR Biosciences) in Odyssey Blocking Buffer with 0.2% Tween 20. Proteins were visualized using an Odyssey CLx infrared imaging system, and bands were quantified using the Image Studio software (LI-COR Biosciences).
Primary antibodies used included mouse monoclonal anti-GluA1 (1:1,000; MAB2263, Millipore, Temecula, CA); rabbit polyclonal anti-phospho-Ser845-GluA1 (1:1,000; AB5849, Millipore), mouse monoclonal anti-phospho-(Thr202/Tyr204)- p44/42 ERK1/2 (1:1000; 9106s, Cell Signaling), rabbit polyclonal anti-p42/44 ERK1/2 (1:5000; 9102s, Cell Signaling), and mouse monoclonal anti-α-tubulin (1:10,000; T6199, Sigma-Aldrich).
For data analysis, phospho-proteins (pERK1, pERK2, pSer845-GluA1) were normalized to corresponding total protein/tubulin and total proteins (ERK1, ERK2, Glua1) were normalized to tubulin.
Results
Morphine CPP
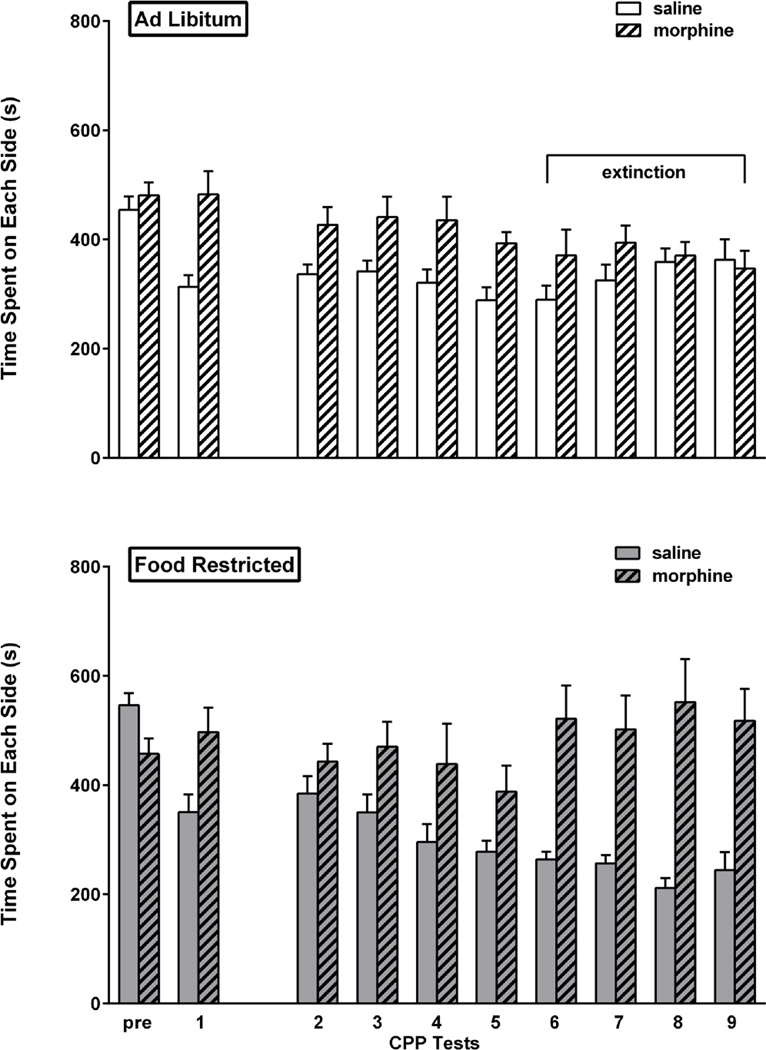
Prior to conditioning (Pre), the eighteen rats in this experiment showed no unconditioned preference for either side of the place preference apparatus. In the first post-conditioning expression test, there was a marked preference for the morphine-paired side of the chamber (t(17)=4.64, p<.001). This result was followed by establishment of two diet groups matched for CPP expression (group assigned to food restriction: t(8)=3.09, p<.01; group assigned to ad libitum feeding: t(8)=3.29, p<.01) and side of chamber associated with morphine, and was followed by a three week hiatus during which the group assigned to food restriction attained and stabilized at target body weight. Following the resumption of testing, CPP extinguished in the ad libitum fed group after the fifth test, as defined by absence of a morphine side preference on the 6th, 7th, 8th and 9th test days. The food restricted group continued to display a CPP throughout this period, including the 6th (t(8)=4.05, p<.01), 7th (t(8)=3.78, p<.01), 8th (t(8)=4.01, p<.01), and 9th (t(8)=3.82, p<.01) test days. In a direct comparison of the two feeding groups, based on CPP difference scores via 2-way mixed design ANOVA (diet × day), the difference between diet in the final three tests was confirmed (F(1,16)= 15.3, p<.002) with no effect of test day, and no interaction between diet and test day. Results are displayed in Figure 1.
Figure 1. Morphine CPP.
Mean (± s.e.m.) time spent (sec) on the morphine- and saline-paired sides of the CPP apparatus during the pre-conditioning session (pre), the first post-conditioning test session (1) and all tests after the three week hiatus (2–9) in ad libitum fed (n=9; top) and food-restricted (n=9; bottom) rats. Testing continued until the ad libitum fed group satisfied extinction criterion (sessions 6–9 as indicated by horizontal bracket).
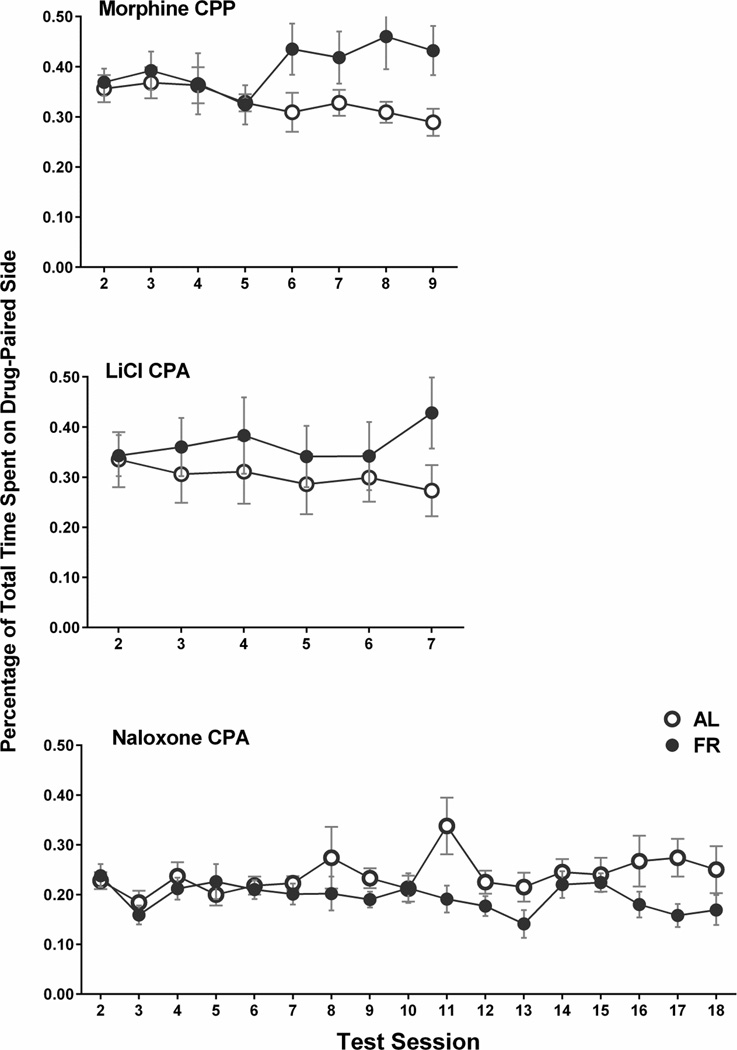
Considering that changes in the balance of time spent between the vehicle-paired side and the neutral center may contribute to the difference score as calculated above, and to facilitate comparison of the three experiments in this study, time spent in the morphine-paired compartment across test sessions was examined. Figure 10 (top) indicates that divergence between diet groups (final three test sessions: F(1,16)=6.32, p<.025) results from a combination of progressive decrease in the ad libitum fed group and increase in the food restricted group. Interestingly, food restricted subjects displayed a significant linear trend of increasing time spent on the morphine-paired side with repeated testing (F(1,56)=17.3, p<.005). This same trend was seen in food restricted subjects expressing a cocaine CPP (Zheng et al. 2012).
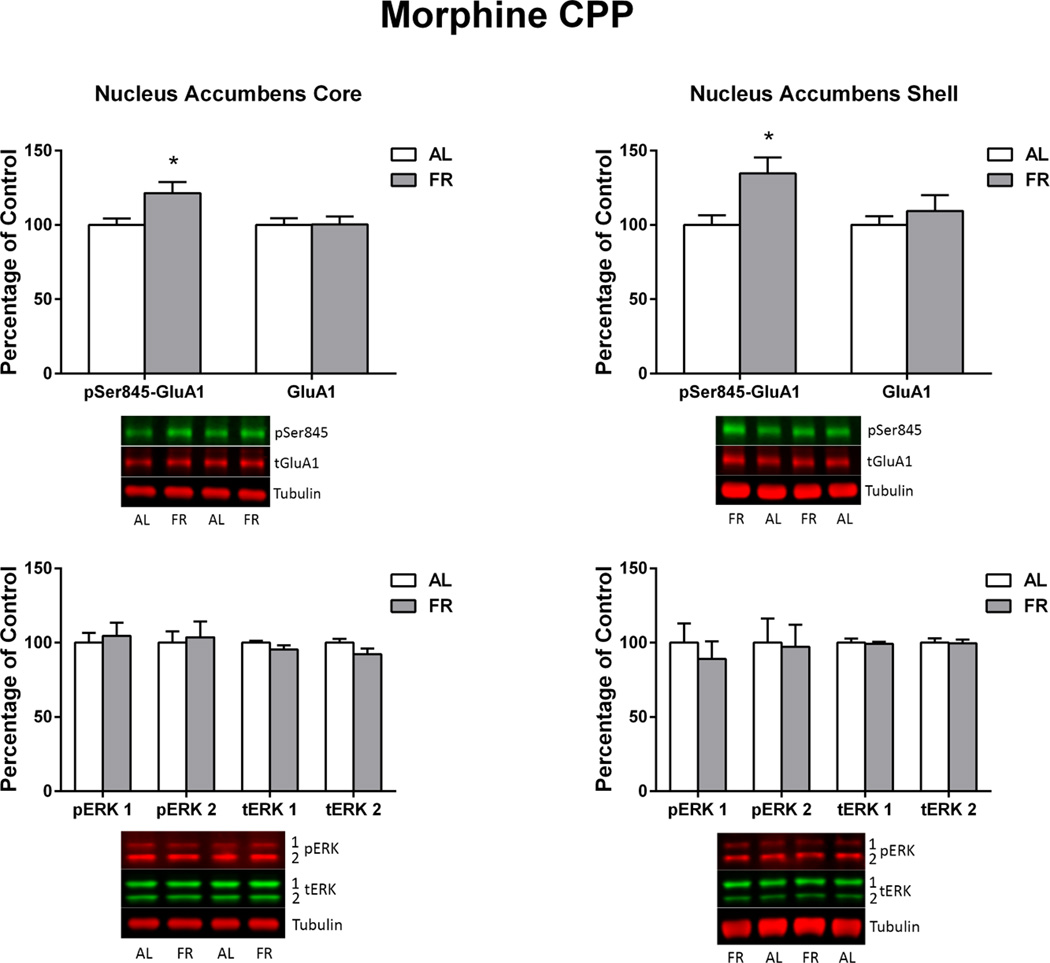
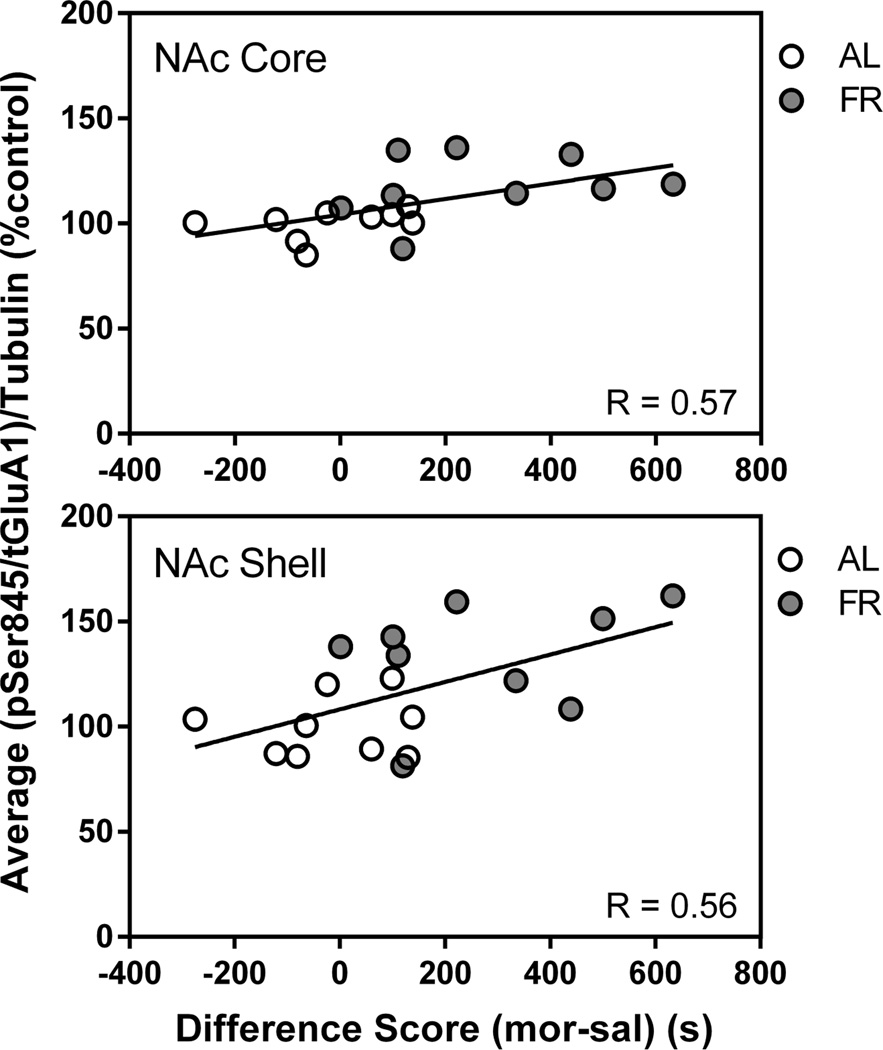
Immediately following the final behavioral test, in which the food restricted group displayed a robust CPP and the ad libitum fed group did not, levels of pSer845-GluA1 in the NAc core and shell of food restricted rats were higher than in ad libitum fed rats (core: t(16)=2.39, p<.05; shell: t(16)=2.62, p<.02). This difference was not seen in CPu. There were no differences between diet groups in levels of total GluA1, pERK1 or pERK2 (Figure 2). Among all subjects, regression analyses indicated a positive correlation between individual subjects’ difference scores (time spent in the morphine-paired compartment – time spent in the saline-paired compartment) and phosphorylation of GluA1 at Ser845 in the core (r=.58 t(16)=2.83, p<.02) and shell (r=.56; t(16)=2.7, p<.02; Figure 3). These correlations are mainly reflective of the diet group differences, in as much as regression analyses confined to individual diet groups were positive but nonsignificant. This differs from previous cocaine CPP studies in which this correlation in NAc core was significant within each diet group (Zheng et al. 2013, 2015). There were no significant correlations between total GluA1, pERK1, or pERK2 and behavior.
Figure 2. Morphine CPP.
Levels of pSer845-GluA1, pERK1, pERK2 and corresponding total proteins in nucleus accumbens core (left) and shell (right) of ad libitum fed (AL) and food-restricted (FR) rats. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed (AL) group. Representative immunoblots are included beneath each bar graph. *p<.05
Figure 3. Morphine CPP.
Scatter plots: correlation between pSer845-GluA1 in NAc core (top) and shell (bottom) and morphine CPP difference score in the final test session among all subjects in the experiment.
LiCl CPA
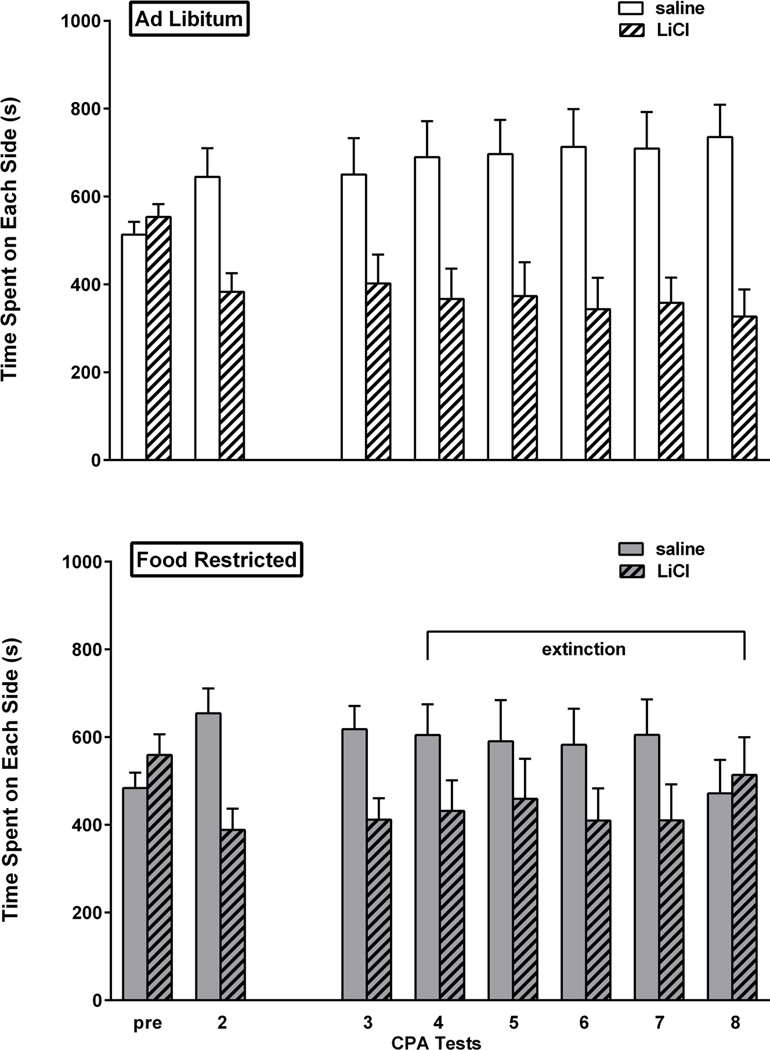
Prior to conditioning (Pre), the twenty four rats in this experiment showed no unconditioned preference for either side of the place preference apparatus. In the first post-conditioning expression test, rats displayed a strong aversion to the LiCl-paired side of the chamber (t(23)=3.65, p<0.001). Using these results, two groups were matched on CPA expression and side of chamber associated with LiCl. The resulting groups both displayed CPA (ad libitum fed: t(11)=2.46, p<0.05; food restricted: t(11)=2.59, p<0.05). This was followed by a three week hiatus during which the group assigned to food restriction attained and stabilized at target body weight. Upon the resumption of testing, extinction criterion was met by the food restricted group after the third test, defined by absence of a CPA on the 4th, 5th and 6th test sessions. CPA was similarly absent in the subsequent test and then on the day of brain harvesting. In contrast, the ad libitum fed group continued to display a CPA in tests 4 through 8 (t(11)=2.2; t(11)=2.1; t(11)=2.4; t(11)=2.6; t(11)=3.1; all p<.05). In a direct comparison of the two feeding groups, based on CPA difference scores via 2-way mixed design ANOVA (diet × day), the difference between diet groups in the final three tests was confirmed (F(1,22)= 4.25, p=.05) with no effect of test day and no interaction between diet and test day. Results are displayed in Figure 4.
Figure 4. LiCl CPA.
Mean (± s.e.m.) time spent (sec) on the LiCl- and saline-paired sides of the CPA apparatus during the pre-conditioning session (pre), the first post-conditioning test session (1) and all tests after the three week hiatus (3–8) in ad libitum fed (n=12; top) and food-restricted (n=12; bottom) rats. Testing continued until the food-restricted group satisfied extinction criterion (sessions 4–8 as indicated by horizontal bracket).
Examination of time spent in the LiCl-paired side, irrespective of time spent elsewhere (Figure 10, middle), suggests that diet groups diverged beginning with the second test following the return from three week hiatus, though on this measure the difference between groups across the final three test sessions was not statistically significant.
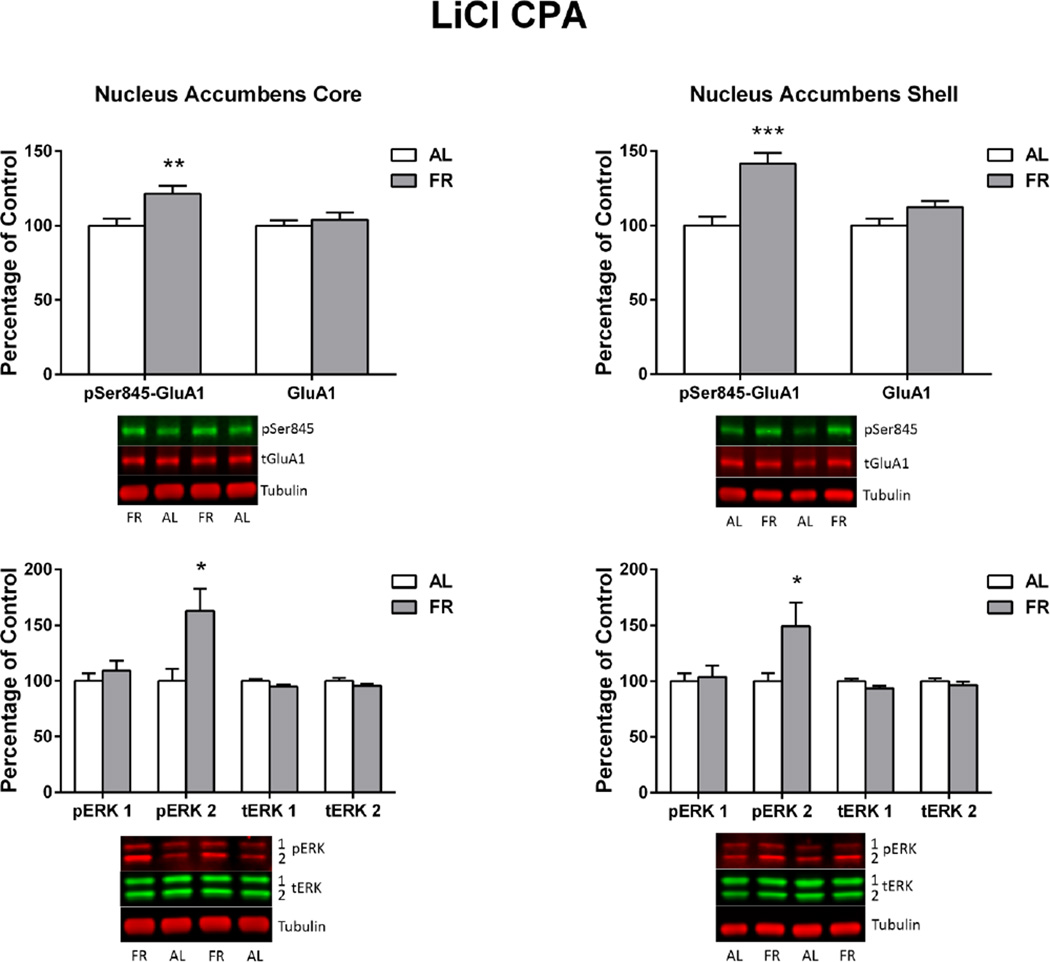
Immediately following the final behavioral test, in which the ad libitum fed group displayed a CPA and the food restricted group did not, levels of pSer845-GluA1 in the NAc core and shell of food restricted rats were higher than in ad libitum fed rats (core: t(22)=3.06, p<.01; shell: t(22)=4.35, p<.0001). Levels of pERK2 were also higher in NAc core (t(22)=2.77, p<.02) and shell (t(22)=2.18, p<.05; Figure 5) of food restricted relative to ad libitum fed rats. These differences were not seen in CPu. There were no differences between diet groups in levels of total GluA1 or pERK1, and no significant correlations between any biochemical measure in any brain region and CPA difference scores.
Figure 5. LiCl CPA.
Levels of pSer845-GluA1, pERK1, pERK2 and corresponding total proteins in nucleus accumbens core (left) and shell (right) of ad libitum fed (AL) and food-restricted (FR) rats sacrificed immediately after the final LiCl CPA test. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed (AL) group. Representative immunoblots are included beneath each bar graph. **p<.01, *p<.05
Naloxone-precipitated withdrawal CPA
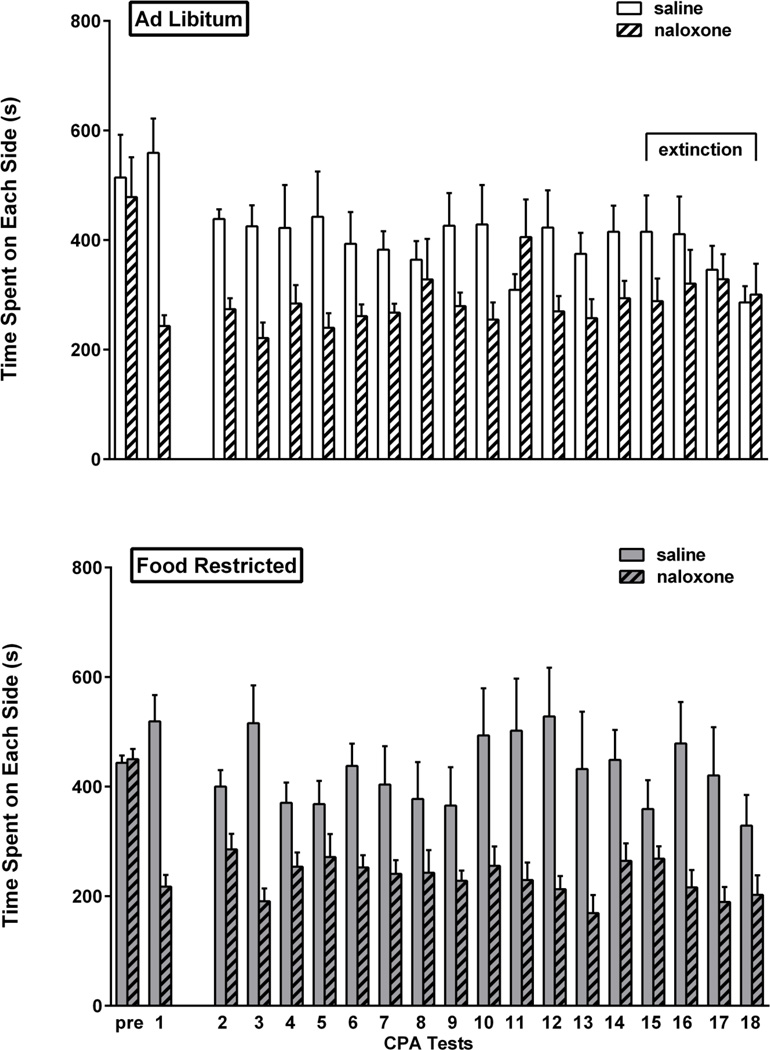
Prior to conditioning (Pre), the 17 rats in this experiment showed no unconditioned preference for either side of the place preference apparatus. In the first post-conditioning expression test, rats displayed a strong aversion to the naloxone-paired side of the chamber (t(16)=7.66, p<.001). Using these results, two groups were matched on CPA expression and side of chamber associated with naloxone. The resulting groups both displayed CPA (ad libitum fed: t(7)=4.74, p=.001; food restricted: t(8)=5.86, p<0.001). This was followed by a three week hiatus during which the group assigned to food restriction attained and stabilized at target body weight. Upon the resumption of testing, extinction criterion was met by the ad libitum fed group in sessions 15–17 as well as in the 18th session that preceded brain harvesting. Although food restricted rats did not meet criterion for CPA in session 15, they resumed CPA expression in sessions 16 (t(8) =3.3, p=.005), 17 (t(8)= 2.69, p<.02), as well as in the 18th session that preceded brain harvesting (t(8)=1.93, p<.05). In a direct comparison of the two feeding groups, based on CPA difference scores via 2-way mixed design ANOVA (diet × day), the difference between diet groups in the final three tests was confirmed (F(1,15)= 4.38, p=.053) with no effect of test day and no interaction between diet and test day. Results are displayed in Figure 6.
Figure 6. Naloxone CPA.
Mean (± s.e.m.) time spent (sec) on the naloxone- and saline-paired sides of the CPA apparatus during the pre-conditioning session (pre), the first post-conditioning test session (1) and all tests after the three week hiatus (2–18) in ad libitum fed (n=8; top) and food-restricted (n=9; bottom) rats. Testing continued until the ad libitum fed group satisfied extinction criterion (sessions 15–18 as indicated by horizontal bracket).
Examination of time spent in the naloxone-paired side, irrespective of time spent elsewhere (Figure 10, bottom), suggests that the food restricted group maintained a relatively stable avoidance while the ad libitum fed group tended toward increasing time in the naloxone-paired side after the 7th test session (final three test sessions: F(1,15)=5.68, p<.05).
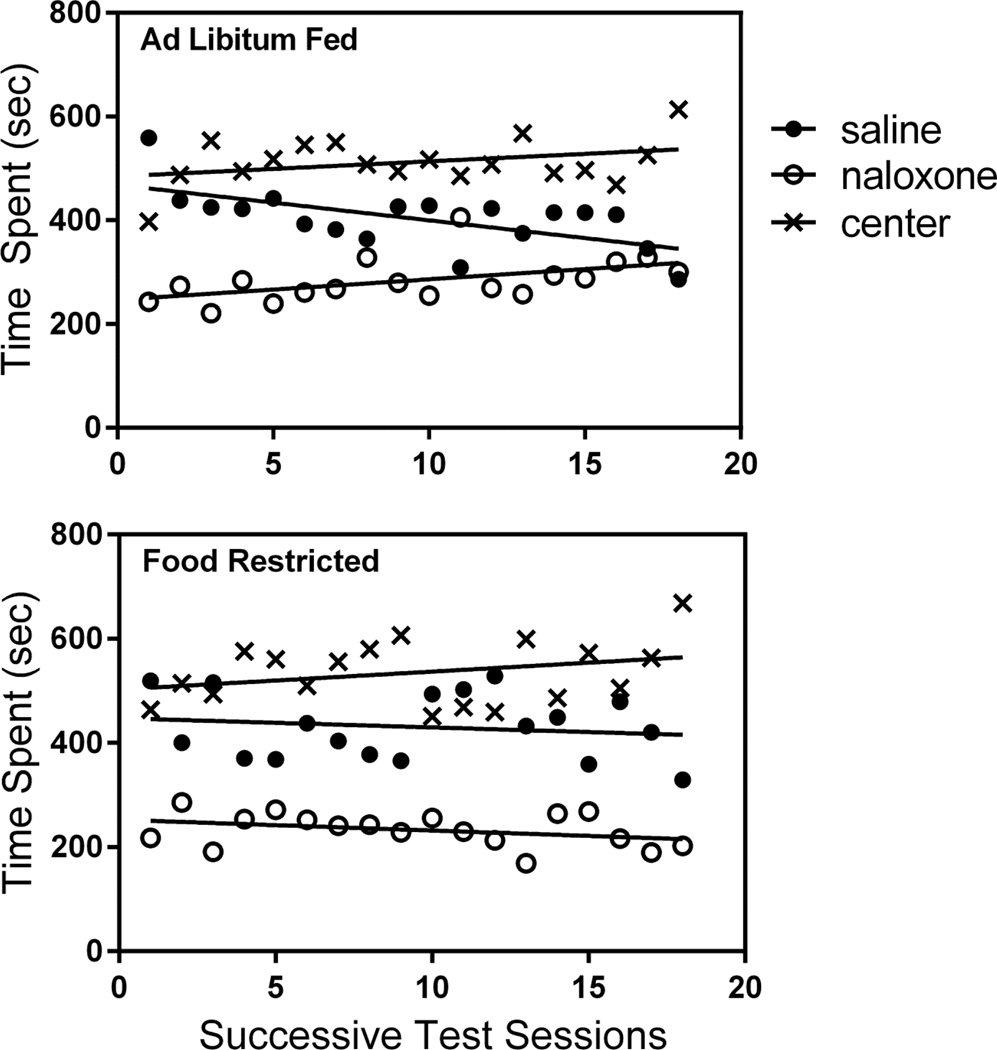
Subjects in the naloxone CPA experiment spent an appreciable portion of their 1200 sec test sessions in the center compartment of the three-compartment apparatus. To more closely evaluate the behavior of these subjects, time spent in each of the three locations are plotted in Figure 7. It is clear that ad libitum fed and food restricted subjects spent equal time in the center with a similar subtle increase over the course of successive test sessions (slopes: ad libitum fed= +2.89; food restricted= +3.42). Importantly, over the course of testing, the food restricted group maintained separation between time spent in the saline- and naloxone-paired sides (slopes: saline= −1.78; naloxone= −2.06) while the ad libitum fed group displayed convergence (slopes: saline= −6.84; naloxone= +3.95), again suggesting CPA extinction in the ad libitum fed but not the food restricted group.
Figure 7. Naloxone CPA.
Scatter plots: average time spent, in seconds, in the saline-paired side, naloxone-paired side, and center compartment of the place conditioning apparatus over the course of 18 successive test sessions by ad libitum fed (top) and food-restricted (bottom) rats.
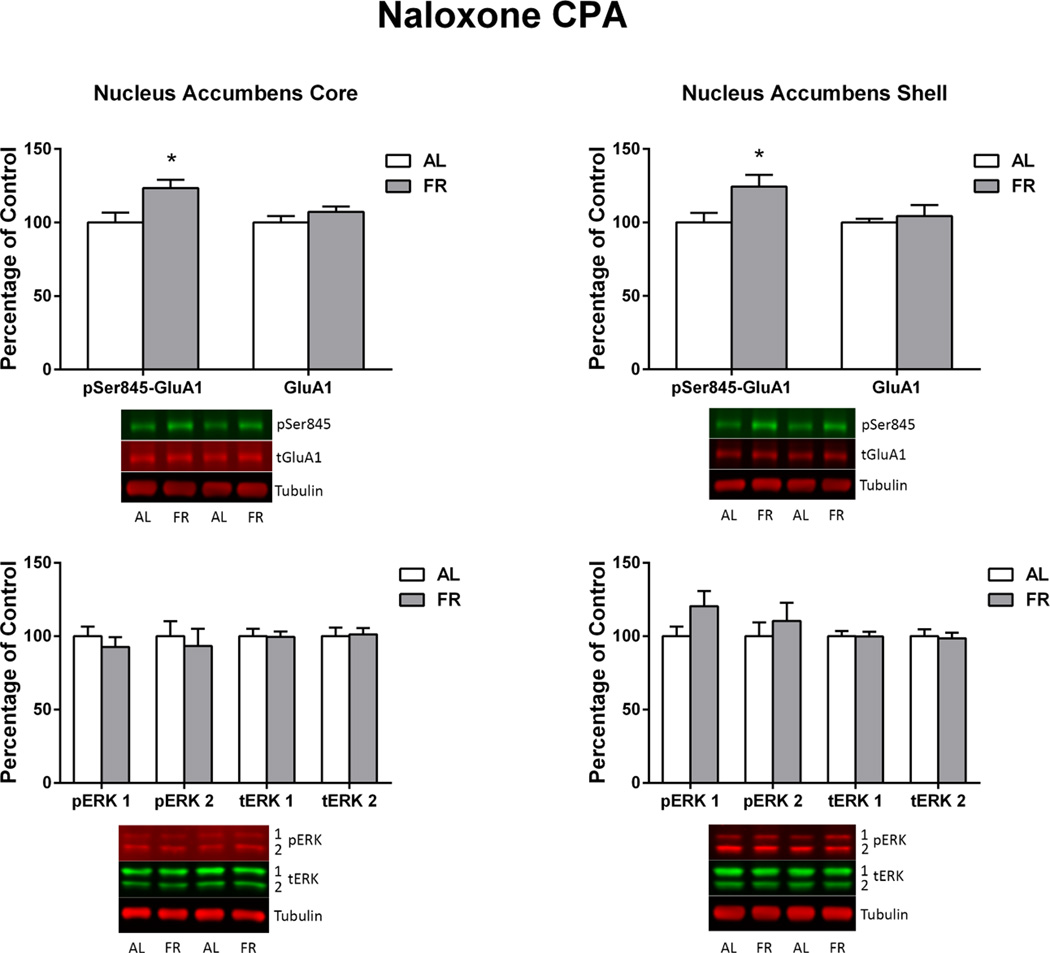
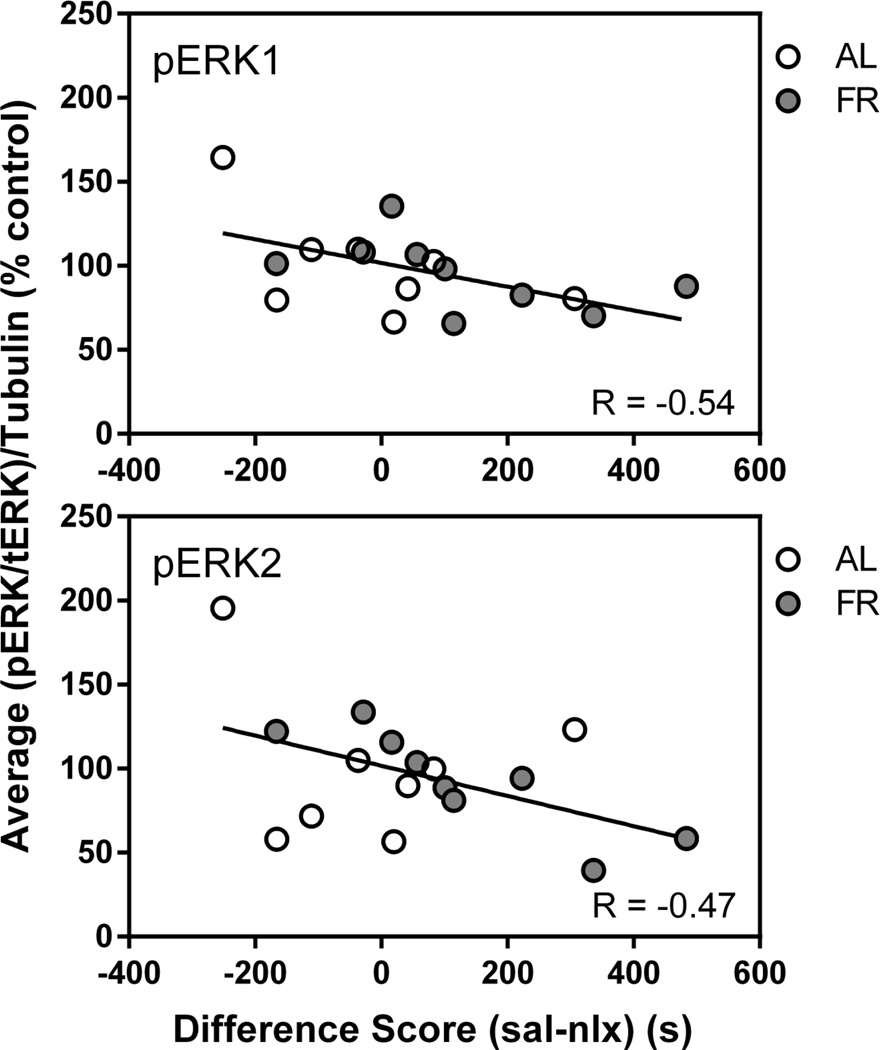
Immediately following the final behavioral test, in which the food restricted group displayed a robust CPA and the ad libitum fed group did not, levels of pSer845-GluA1 in the NAc core and shell of food restricted rats were higher than in ad libitum fed rats (core: t(15)=2.7, p<.025; shell: t(15)=2.29, p<.05; Figure 8). These differences were not seen in CPu. Diet groups did not differ in levels of total GluA1, pERK1 or pERK2 in any brain region. However, among all subjects, regression analyses indicated a negative correlation between difference scores (time spent in the saline-paired compartment – time spent in the naloxone-paired compartment) and pERK1 (r=−.54; t(15)=2.49, p=.025 and pERK2 (r=−.47; t(15)=2.07, p=.056) in NAc core (Figure 9). There were no significant correlations between pSer845-GluA1 or total GluA1 and behavior.
Figure 8. Naloxone CPA.
Levels of pSer845-GluA1, pERK1, pERK2 and corresponding total proteins in nucleus accumbens core (left) and shell (right) of ad libitum fed (AL) and food-restricted (FR) rats sacrificed immediately after the final naloxone CPA test. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed (AL) group. Representative immunoblots are included beneath each bar graph. *p<.05
Figure 9. Naloxone CPA.
Scatter plots: correlation between pERK1 (top) and pERK2 (bottom) in nucleus accumbens core and naloxone CPA difference score in the final test session among all subjects in the experiment.
Figure 10. Time Spent on the Drug-Paired Side.
Percentage of the total test period (mean ± s.e.m.) spent on the drug-paired side of the apparatus, across successive test sessions, by ad libitum fed (open circles) and food restricted (filled circles) subjects in the morphine CPP (top), LiCL CPA (middle), and naloxone CPA (bottom) experiments.
Dependence of increased pSer845-GluA1 on pairing of context with drug injection
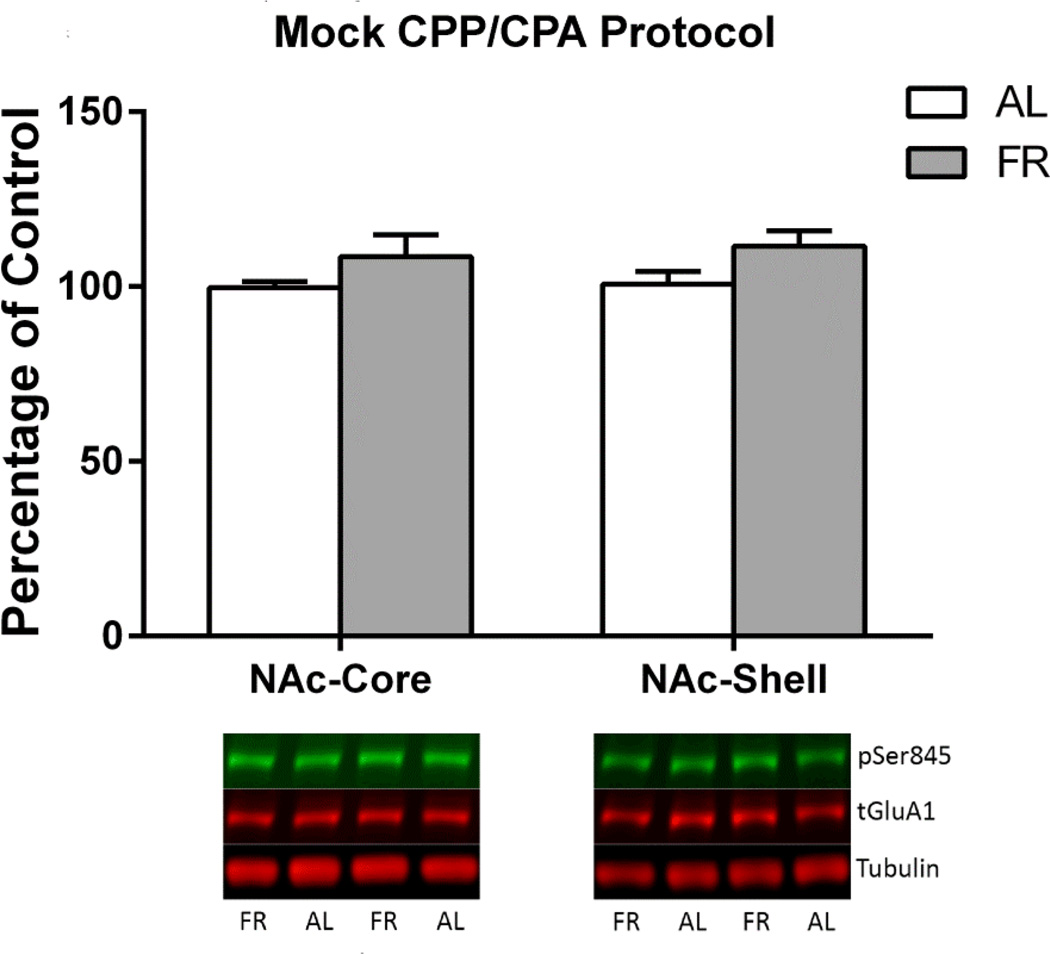
A common finding among the three experiments is elevated pSer845-GluA1 in the NAc of food restricted rats whose brains were harvested immediately after behavioral testing. In order to determine whether these elevations depend on drug-context conditioning, a control experiment was conducted. Eighteen subjects were “conditioned” in 8 sessions with saline injected prior to each session. Nine subjects each were then assigned to ad libitum and food restricted diet treatments. Three weeks later, daily “expression” testing was conducted and brains were harvested immediately after the seventh test. Levels of pSer845-GluA1 did not differ between diet groups (Figure 11).
Figure 11. Mock CPP/CPA Protocol.
Levels of pSer845-GluA1 in nucleus accumbens core (left) and shell (right) of ad libitum fed (AL; n=9) and food-restricted (FR; n=9) rats sacrificed immediately after the final “expression” test in a mock conditioning protocol. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed (AL) group. Representative immunoblots are included beneath each pair of bars.
Discussion
The first aim of the present study was to determine whether the enhancing effect of food restriction on CPP persistence applies to morphine as it does to cocaine. Thus, in Experiment 1 food restriction was implemented after conditioning and confirmation of a morphine CPP. When testing resumed three weeks later, CPP was found to extinguish in subjects that were ad libitum fed throughout the study but continued to be expressed by subjects switched to food restriction. This result matches the result previously obtained with cocaine (Zheng et al. 2012). Interestingly, the divergence between diet groups in both studies reflected not only decreasing time spent on the drug-paired side by ad libitum fed subjects but increasing, rather than stable, time spent by the food restricted subjects. This observation adds weight to the possibility that food restriction enhances the incentive effect of the drug-paired context with repeated exposure, at least for a time, similar to the sensitization that might be seen if the drug itself were being repeatedly administered.
Immediately following the final morphine CPP test, levels of pSer845-GluA1 were higher in NAc core and shell of food restricted relative to ad libitum fed rats, and the magnitude of CPP correlated with levels of pSer845-GluA1. The increase in pSer845-GluA1 appears to be triggered by exposure to the context previously paired with drug administration, rather than the basal state of subjects, because food restricted subjects that underwent “mock” conditioning and testing showed no increase. GluA1 is phosphorylated at Ser845 downstream of D1 dopamine receptor signaling (Snyder et al. 2000) and the upregulated response of food restricted rats to context exposure matches their upregulated response to D1 agonist administration (Carr et al. 2010). Considering that phosphorylation at Ser845 increases AMPA currents, and trafficking and stabilization of GluA1 in the synaptic membrane (Barry and Ziff 2002; He et al. 2009; Kessels and Malinow, 2009; Choquet 2010), the relationship between levels of this phosphorylated AMPA receptor subunit and CPP expression may reflect increased excitation of D1 receptor-expressing medium spiny neurons, known to increase approach and enhance CPP (Lobo et al. 2010).
The second aim of this study was to determine whether the enhancing effect of food restriction extends to conditioned place aversion (CPA). Thus, in Experiment 2 LiCl, which induces visceral illness, taste aversion (Nachman and Ashe 1973), and CPA (Parker and McDonald 2000) was used to condition a CPA in ad libitum fed rats. Following confirmation of CPA expression, subjects were assigned to ad libitum and food restricted diet groups, and CPA testing resumed three weeks later. Interestingly, the effect of food restriction was, if anything, opposite that observed in the CPP studies. After the switch to food restriction, subjects displayed a weak CPA that extinguished rapidly, while subjects that remained ad libitum fed continued to display a CPA. Despite this, food restricted subjects again displayed elevated levels of pSer845-GluA1 in NAc core and shell. In addition, pERK2 levels were markedly higher in NAc core and shell of food restricted relative to ad libitum fed rats. Both of these biochemical findings are curious given that ERK phosphorylation in NAc core is necessary for expression of a cocaine CPP (Miller and Marshall 2005b) as well as reconsolidation of both cocaine and morphine CPP (Miller and Marshall 2005b; Valjent et al. 2006). Moreover, pERK plays a role in maintaining NAc synaptic incorporation of GluA1-containing AMPARs (Ferrario et al. 2011), including during the reconsolidation of a morphine CPP (Lv et al. 2015). The current methods do not enable determination of whether increased pERK2 and pSer845-GluA1 occur in a common population of medium spiny neurons. However, if co-expression is assumed, there is the seeming paradox that biochemical findings in food restricted rats should favor increased expression of a conditioned response. Having said that, NAc involvement in CPA expression is not as well investigated as CPP, and fundamental differences have been reported. For example, NMDA receptors in NAc mediate reconsolidation of morphine CPP but not naloxone CPA (Wu et al. 2012), and unlike cocaine CPP (Miller and Marshall 2005b), blockade of ERK phosphorylation does not decrease expression of LiCl CPA (Longoni et al. 2011). In fact, contrary to the positive role of ERK phosphorylation in CPP expression, ERK phosphorylation in multiple forebrain regions has been associated with CPA extinction (Wang et al. 2012; Wang et al. 2015).
While the role of pSer845-GluA1, pERK2, and any mechanistic relationship between the two in LiCl CPA cannot be resolved based on the current data, it is of interest that food restriction exerted opposite behavioral effects on cocaine/morphine CPP and LiCl CPA. This raises the possibility that food restriction facilitates reward and diminishes aversion, particularly in relation to food, proxies for food (i.e., drugs of abuse), and impediments to feeding. Under conditions of food scarcity, a more enduring behavioral response to a reward-associated context coupled with increased “risk-taking”, as in returning to an environment associated with visceral illness, may have adaptive value by increasing the probability of food acquisition. Parsimony might then point to increased pSer845-GluA1 as a “switch” that alters the balance between reward-directed and avoidant behavior. This idea receives support from findings that blockade of GluA1-containing/GluA2-lacking AMPA receptors in NAc reverses the enhanced rewarding effect of d-amphetamine in food restricted rats (Peng et al. 2014) and exacerbates the pain and depression associated with spared nerve injury (Goffer et al. 2013). A caveat to this proposed scheme is the finding that viral vector mediated overexpression of GluA1 in NAc shell decreases rather than increases brain stimulation reward (Todtenkopf et al. 2006). Reconciliation of these seeming contradictory findings will likely require identification of affected neuronal phenotype(s) in NAc (Lobo et al. 2010; Kravitz et al. 2012) and characterization of changes in AMPA receptor subunit abundance in the postsynaptic density.
In Experiment 3, food restriction increased persistence of the naloxone CPA and was accompanied by increased pSer845-GluA1 in NAc core and shell, plus an overall negative correlation between CPA and pERK1/pERK2 levels in NAc core. These results militate against a scheme in which food restriction generally shifts the balance between reward and aversion. Differential effects of food restriction on LiCl and naloxone CPA could be explained by the substantially different neuroanatomical patterns of neuronal activation induced by these two aversive stimuli (Gracy et al. 2001; Ferreira et al. 2006) or, to the extent that both are regulated by NAc, the balance between phosphorylation of GluA1 and ERK. If increased pSer845-GluA1 across experiments is to be assigned a consistent function, it may relate to enhanced recognition of stimulus salience or responsiveness to the context with positive valence but, in the case of CPA, is subject to override by ERK phosphorylation that mediates extinction learning. Thus, in LiCl CPA, the robust increase in pERK2 prevailed and CPA extinguished, while in naloxone CPA, increased pSer845-GluA1 prevailed but in inverse relation to the level of pERK. Understanding the relationship between changes in pSer845-GluA1 and pERK as they relate to CPP vs. CPA and their modulation by food restriction may depend not only on localization of these molecular changes within cells and NAc subregions, but also between subregions. For example, in contrast to the key involvement of NAc core in CPP expression (Miller and Marshall 2005a,b; Theberge et al. 2010), a glutamatergic projection from paraventricular thalamus to NAc shell mediates expression of a naloxone CPA (Li et al. 2011).
From a behavioral standpoint, what is clear based on present results and prior results with cocaine, is that food restriction does not enhance expression of all forms of place conditioning but, so far, has increased the persistence of place conditioning induced by administration and withdrawal from drugs of abuse. Given the importance of cues and contexts as triggers of craving and relapse, these findings add further support to the contention that food restriction and key CNS concomitants not only increase vulnerability to initial use by enhancing drug reward magnitude, but may also increase vulnerability to relapse triggered by exposure to environmental cues and contexts.
Acknowledgments
This research was supported by DA03956 from NIDA/NIH.
References
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neuroscience. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O'Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Choquet D. Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur J Neurosci. 2010;32:250–260. doi: 10.1111/j.1460-9568.2010.07350.x. [DOI] [PubMed] [Google Scholar]
- Collins GT, Chen Y, Tschumi C, Rush EL, Mensah A, Koek W, France CP. Effects of consuming a diet high in fat and/or sugar on the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice. Exp Clin Psychopharmacol. 2015;23:228–237. doi: 10.1037/pha0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cunha TM, Sedki F, Macri J, Casola C, Shalev U. The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacology. 2013;225:241–250. doi: 10.1007/s00213-012-2810-1. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2þ-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacol. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G, Ferry B, Meurisse M, Levy F. Forebrain structures specifically activated by conditioned taste aversion. Behav Neurosci. 2006;120:952–962. doi: 10.1037/0735-7044.120.4.952. [DOI] [PubMed] [Google Scholar]
- Goffer Y, Xu D, Eberle SE, D’amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci. 2013;33:19034–19044. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of condtioned place aversion. Neuropsychopharmacol. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, Kirouac GJ. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav. 2011;102:42–50. doi: 10.1016/j.physbeh.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni R, Spina L, Vinci S, Acquas E. The MEK inhibitor SL327 blocks acquisition but not expression of lithium-induced conditioned place aversion: a behavioral and immunohistochemical study. Psychopharmacology. 2011;216:63–73. doi: 10.1007/s00213-011-2192-9. [DOI] [PubMed] [Google Scholar]
- Lv XF, Sun LL, Cui CL, Han JS. NAc shell Arc/Arg3.1 protein mediates reconsolidation of morphine CPP by increased GluR1 cell surface expression: Activation of ERK-coupled CREB is required. Int J Neuropsychopharmacol. 2015;18(9) doi: 10.1093/ijnp/pyv030. pii: pyv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005a;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005b;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10:73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Parker LA, Joshi A. Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav. 1998;61:331–333. doi: 10.1016/s0091-3057(98)00104-x. [DOI] [PubMed] [Google Scholar]
- Parker LA, Mcdonald RV. Reinstatement of both a conditioned place preference and a conditioned place aversion with drug primes. Pharmacol Biochem Behav. 2000;66:559–561. doi: 10.1016/s0091-3057(00)00222-7. [DOI] [PubMed] [Google Scholar]
- Peng X-X, Cabeza de Vaca S, Ziff EB, Carr KD. Involvement of nucleus accumbens AMPA receptor trafficking in augmentation of D- amphetamine reward in food-restricted rats. Psychopharmacology. 2014;231:3055–3063. doi: 10.1007/s00213-014-3476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behav Neurosci. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedki F, Gardner Gregory J, Luminare A, D'Cunha TM, Shalev U. Food restriction-induced augmentation of heroin seeking in female rats: manipulations of ovarian hormones. Psychopharmacology. 2015;232:3773–3782. doi: 10.1007/s00213-015-4037-4. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg A, Valee CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Kim R, Cho J, Michaelides M, Anderson BJ, Primeaux SD, Bray GA, Wang GJ, Robinson JK, Volkow ND. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rats. Physiol Behav. 2010;101:713–718. doi: 10.1016/j.physbeh.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, Carlezon WA., Jr Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J Neurosci. 2006;26:11665–11669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U.S.A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WS, Chen ZG, Liu WT, Chi ZQ, He L, Liu JG. Dorsal hippocampal NMDA receptor blockade impairs extinction of naloxone precipitated conditioned place aversion in acute morphine-treated rats by suppressing ERK and CREB phosphorylation in the basolateral amygdala. Brit J Pharmacol. 2015;172:482–491. doi: 10.1111/bph.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, Chen J, Chi ZQ, He L, Liu JG. Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci. 2012;32:13763–13775. doi: 10.1523/JNEUROSCI.1991-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li Y, Gao J, Sui N. Differential effect of NMDA receptor antagonist in the nucleus accumbens on reconsolidation of morphine-related positive and aversive memory in rats. Eur J Pharmacol. 2012;674:321–326. doi: 10.1016/j.ejphar.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Zheng D, Cabeza de Vaca S, Carr KD. Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacol Biochem Behav. 2012;100:538–544. doi: 10.1016/j.pbb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liu S, Cabeza de Vaca S, Carr KD. Effects of time of feeding on psychostimulant reward, conditioned place preference, metabolic hormone levels, and nucleus accumbens biochemical measures in food-restricted rats. Psychopharmacology. 2013;227:307–320. doi: 10.1007/s00213-013-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Cabeza de Vaca S, Jurkowski Z, Carr KD. Nucleus accumbens AMPA receptor involvement in cocaine-conditioned place preference under different dietary conditions in rats. Psychopharmacology. 2015;232:2313–2322. doi: 10.1007/s00213-015-3863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]