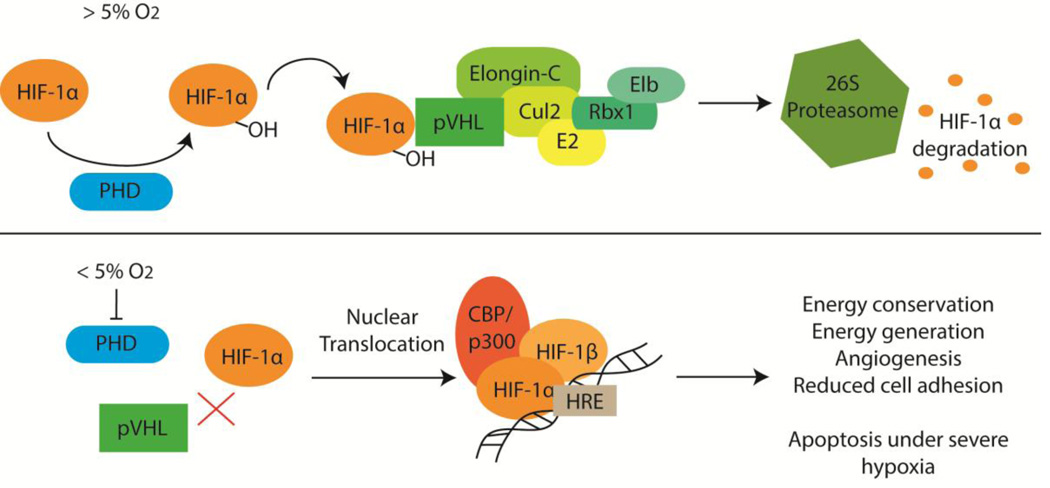
Figure 1.
The hypoxia inducible factor 1 α-subunit (HIF-1α) is constitutively expressed. When the cellular oxygen tension is above 5%, HIF-1α is degraded. This is initiated when prolyl-4-hydroxylases (PHD) add hydroxyl (-OH) groups to HIF-1α. The von-Hippel Lindau protein (pVHL) binds hydroxylated HIF-1α and recruits other proteins to aid in the ubiquitinylation of HIF-1α, targeting it for degradation by the 26S proteasome. When the oxygen tension drops below 5%, PHDs can no longer hydroxylate HIF-1α, preventing pVHL from binding. HIF-1α accumulates in the cytosol and binds the HIF-1β/ARNT subunit. This heterodimeric transcription factor complex binds the CBP/p300 coactivator and this complex then translocates to the nucleus where it binds hypoxia-responsive elements (HRE) throughout the genome to up-regulate transcription of many genes involved in various processes.