Figure 7.
Osx+ Cell Produces IL-7 and IGF-1 to Regulate B Cell Differentiation
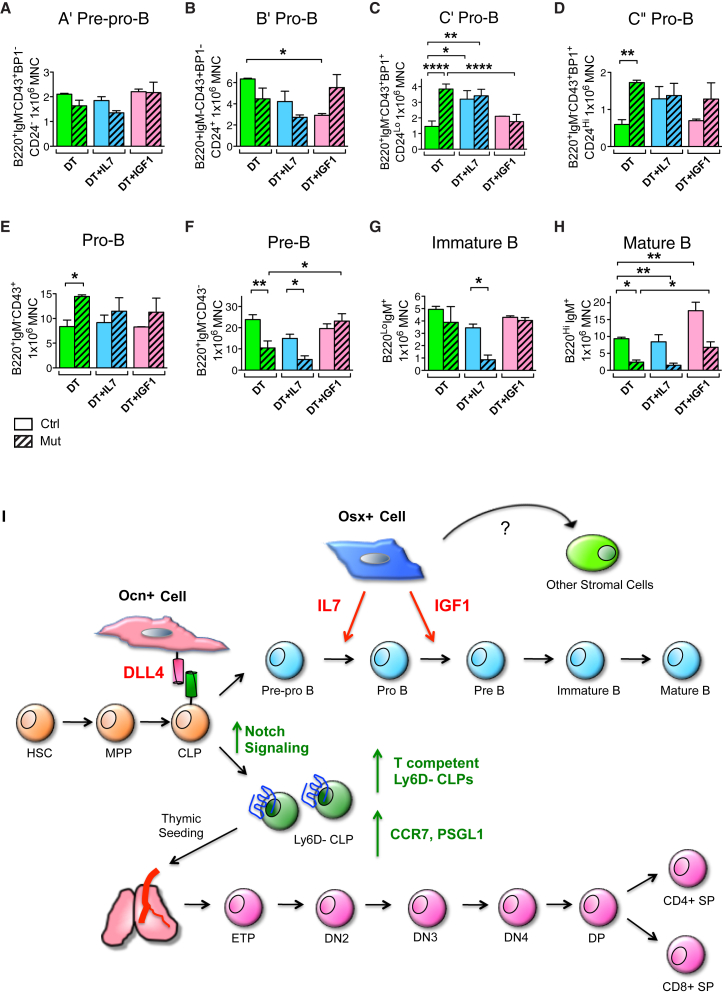
(A–H) In vivo rescue experiment of OsxCre;iDTR lymphoid phenotype. OsxCre;iDTR control and mutant animals were subjected to either: DT, DT + IL-7, or DT + IGF-1 daily injections for 12 days. Bone marrow cells were harvested the next day following the last injection for flow cytometric analysis of B cell differentiation. Injection of IL-7 augmented the base level of C′ and C″ pro-B (C and D), but failed to rescue pre-B and downstream B cell maturation (F–H). In contrast, IGF-1 had no effect on earlier B cell differentiation (A–E), but rescued the pre-B and mature B cell loss in the mutant group (F and H). Three independent experiments; n = 6–9/group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
(I) Model of regulation of B and T lymphopoiesis by Osx+ and Ocn+ osteolineage cells. Osx+ cell produces IL-7 and IGF-1, and both molecules are required to support full B lineage differentiation. While IL-7, also likely produced by other stomal cell types, mediates hematopoietic differentiation from early B cell precursors to pre-B cells, Osx+ cell secretion of IGF-1 is necessary for further B cell maturation from pre-B to mature B. In comparison, Ocn+ cell expresses the Notch ligand DLL4, which binds to cell-surface Notch receptor on the T cell-competent Ly6D− CLP population (Yu et al., 2015a). This engagement ensures T cell progenitor production, and expression of the chemotactic molecules CCR7 and PSGL1 for subsequent thymic seeding. The precise balance of Osx+ and Ocn+ osteolineage cells within the bone marrow niche is critical for both B and T lymphopoiesis, as perturbation of this balance leads to specific loss of B or T immune cells.
Error bars represent ± SEM.