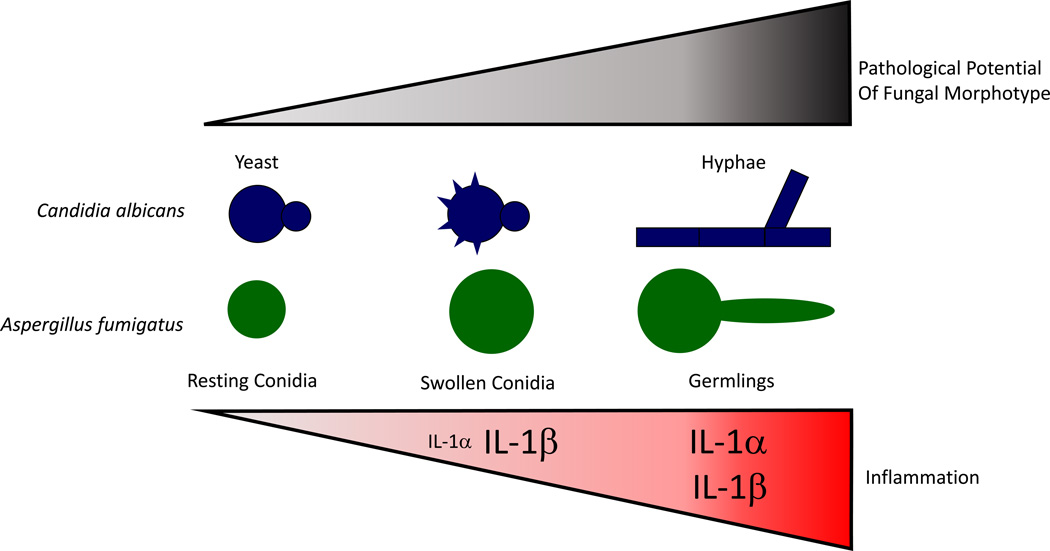
Figure 1. Escalating IL-1 inflammatory response regulates mammalian resistance to invasive, pathological fungal infection.
In homeostatic conditions when the barrier to mucosal surfaces is intact, C. albicans and A. fumigatus can be found in a resting state on mucosal surfaces and in the airways, respectively. Once this primary immune barrier is breached, fungi can access nutrients necessary for growth. In the case of C. albicans, rearrangement of the cell wall occurs during phagocytosis, while in the case of Aspergillus spp. the cell wall architecture is changed upon conidial swelling. In either case, fungal MAMPs are revealed to the immune system resulting in the activation of an inflammasome-dependent immune responses to clear the fungal threat. If the fungi continue to grow, forming invasive hyphae and expressing hyphal-specific effectors, including GAG, secondary metabolites, and proteases, robust tissue pathology can result. This increased pathology drives elevated levels of pro-inflammatory cytokines and alarmin release, such as IL-1α, which intensify the innate immune response in hope of clearing the infection. Font size for each cytokine is indicative of their relative abundance.