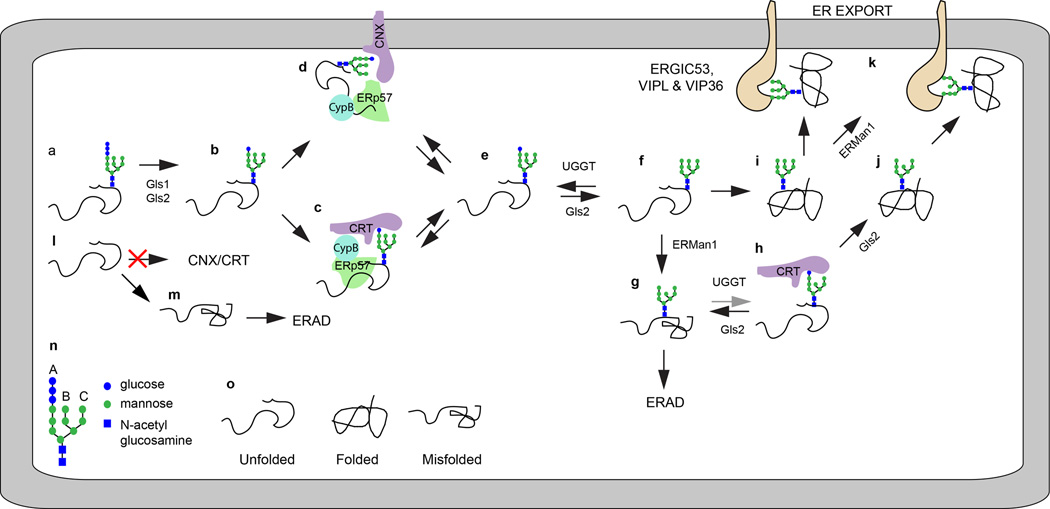
Figure 1.
Asparagine linked glycosylation allows glycoprotein entry into the CNX/CRT cycle. (a–d) GN2M9G1 dependent binding of unfolded proteins to CNX and CRT. For simplicity, the interaction of ERp57 and CypB with CNX and CRT is only depicted in diagrams c and d. (e, f) Gls2 and UGGT promote cyclic binding and release of glycoproteins form CNX (d) and CRT(c). (g,h) ERMan1 cleavage of the B-branch mannose residue reduces the reglucosylation of the glycan by UGGT. (i, j) Folded glycoproteins that exit the CNX/CRT cycle are trimmed by ERMan1 to yield protein linked GN2M8–9. (k) Secretory cargo proteins can be recognized by the lectins ERGIC53, VIP36 and VIPL. Non-glycosylated glycoproteins (l) do not enter the CNX/CRT cycle and are prone to misfolding (m). (n) The diagram shows the symbol code for saccharide residues in GN2M9G3, and indicates the location of the A, B and C branches. (o) The folding status of glycoproteins in diagrams a–m. The gray arrow in diagram h indicates that the pathway shown is less favored relative to that shown with black arrows.