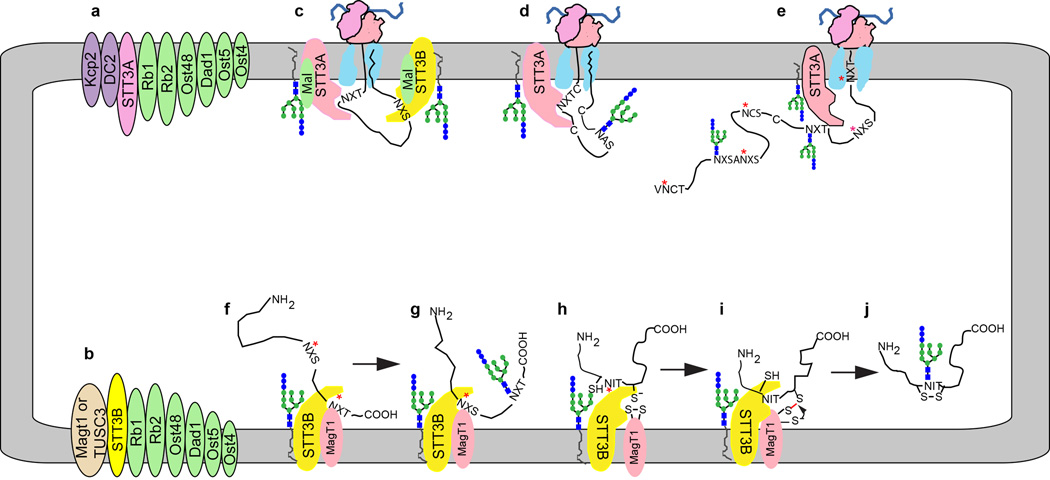
Figure 3.
Cooperation between the STT3A and STT3B complexes maximizes protein glycosylation efficiency. (a, b) Subunit composition of the STT3A and STT3B complexes. Ribophorin 1, ribophorin 2 and TMEM258 are abbreviated as Rb1, Rb2 and Ost5, respectively. (c) Cotranslational translocation of a nascent glycoprotein through the Sec61 protein translocation channel allows cotranslational glycosylation of acceptor sequons by the STT3A complex. The malectin-OST association is only shown in this diagram for simplicity. (d) Cotranslational glycosylation of cysteine rich proteins before disulfide bond formation. (e) Examples of acceptor sequons that have been skipped by the STT3A complex including extreme N and C-terminal sites, closely spaced NXS sites, cysteine proximal sites (NCS) and a poorly understood class of non-optimal sequons (designated as NXS). (f, g) Posttranslocational glycosylation of extreme C-terminal sequons (red asterisks) does not occur by an N to C-terminal scanning mechanism but is instead determined by the relative affinity of the STT3B active site for NXT and NXS sequons. (h–j) Posttranslocational glycosylation of cysteine proximal sequons by the STT3B complex can be facilitated by reversible formation of a mixed disulfide between MagT1 (or TUSC3) and the unfolded glycoprotein. Red asterisks in diagrams e–h indicate skipped acceptor sites.