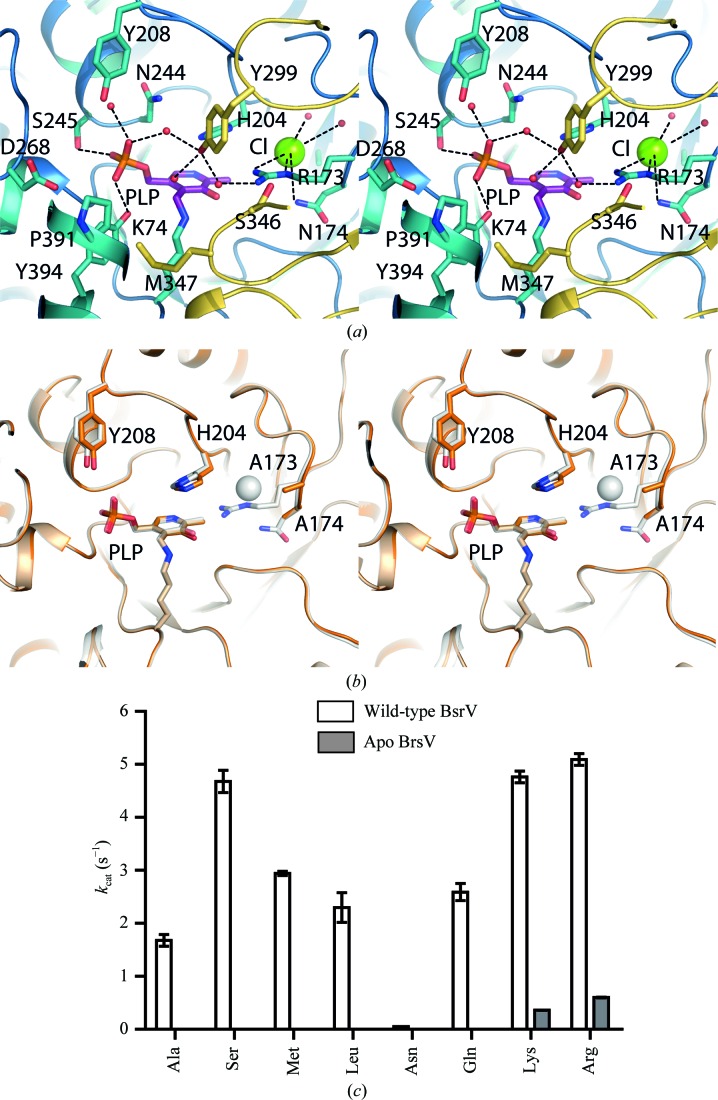
Figure 3.
BsrV and ΔCl-BsrV active-site structures and kinetic parameters. (a) Stereoview of the BsrV active site. Relevant residues and PLP are shown as sticks; water molecules and Cl− ions are shown as red and green spheres, respectively. (b) Stereoview showing the structural superimposition of the active sites of wild-type BsrV (shown as a white transparency) and ΔCl-BsrV (R173N174/AA) (coloured orange). No other significant changes apart from the double mutation and consequent Cl− loss (white sphere) are apparent. (c) Comparison between the k cat of ΔCl-BsrV and BsrV for different racemizable amino acids. The results in (c) are means ± SD of triplicates from two independent experiments. See also Supplementary Fig. S3.