FIG 6.
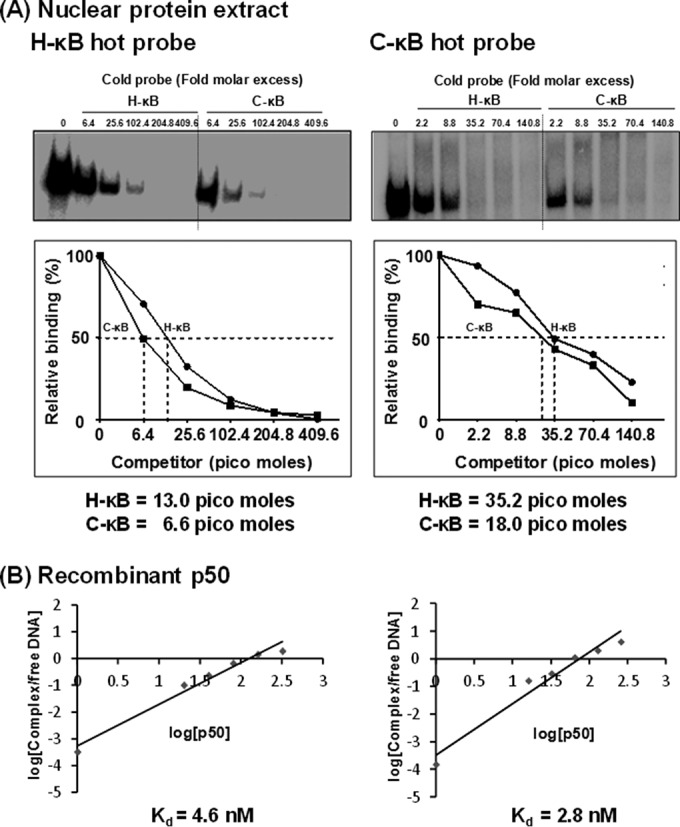
C-κB probe demonstrates a higher binding affinity for NF-κB in the Jurkat nuclear extract as well as recombinant p50 protein. (A) Radiolabeled double-stranded DNA probe (40,000 cpm) comprised of the H-κB or C-κB probe was incubated with 30 μg of the Jurkat nuclear extract. The competition was performed with progressively increasing quantities (fold excess) of H- or C-κB cold probe as shown. (Lower) The intensities of the DNA complexes were determined by densitometry. The percentage of the DNA band intensity was plotted against the concentration of competitor probe. The intercept on the x axis is considered the affinity of binding of the probes for the nuclear factors. The data are representative of three independent experiments. (B) Radiolabeled double-stranded DNA probe (60,000 cpm) comprised of the H-κB or C-κB probe was incubated with 0 to 10.4 nmol of the recombinant p50 protein. The competition was performed with progressively increasing quantities (fold excess) of H- or C-κB cold probe as shown. (Lower) The intensities of the DNA complexes were determined by densitometry. The percentage of the DNA band intensity compared to that of the no-competition control was plotted as log(cold probes) versus log(FP/SC). The intercept on the x axis is considered the affinity of binding of the probes for the nuclear factors. The data are representative of three independent experiments. FP, free probe; SC, shifted complex.
