Abstract
Arabinoxylans (AXs) are an important component of wheat and rye dough. They bind water, contribute to the formation of viscous dough and improve the quality of bread. For the application of AX fractions in bread making process, it is useful to record a quality profile of wheat fractions compared to the quality profile of rye fractions under standardized conditions. In this work water and alkali extractable AX containing fractions, from wheat- and rye wholemeal, were extracted under standardized conditions and characterized. For analysis of composition, structural features, and molecular dimension a combination of chemical, physicochemical, enzymatic and chromatographic techniques was applied. The molar mass distributions obtained by means of an innovative colorimetric pentose detection in the eluted SEC fractions were comparable for all under standardized conditions extracted AXs. The determined molar masses of AXs extracted both from wheat- and from rye grain were close to 2.0 × 105 g/mol for water extractable AXs and 3.0 × 105 g/mol for alkali extractable AXs. Different susceptibility to endoxylanase treatment, having been observed as differences in the SEC profiles, may be evidence of structural differences between AXs depending on their origin. The viscosities of AX solutions were strongly influenced by their molar mass and structure; samples being less susceptible to endoxylanase provided solutions of higher viscosity.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-2135-2) contains supplementary material, which is available to authorized users.
Keywords: Water extractable arabinoxylan, Alkali extractable arabinoxylan, Solubility, Wheat and rye, Molar mass, Viscosity
Introduction
Arabinoxylans (AXs) are non-starch polysaccharides, mainly present in cereal grains predominantly in rye and in smaller amounts in wheat (Vinkx and Delcour 1996). AXs are known for many health benefits and already an intake of 2–10 g AXs per day reduced cholesterol and glucose levels in blood (Girhammar and Nair 1992). Together with starch and gluten AXs deliver significant properties for the dough quality in bread making processes, improving especially its water binding capacity, mechanical properties, stabilization of the gas cells and in consequence providing higher bread volume with excellent crumb structure (Biliaderis et al. 1995; Bushuk 2001; Denli and Ercan 2001; Michniewicz et al. 1990). In the case of rye bread, consumed extensively in eastern Europe, where gluten matrix is missing predominantly the high content of AXs is responsible for the dough quality (Buksa et al. 2010; Bushuk 2001).
The beneficial role of AX fractions in wheat bread is widely known as well. Because of their positive role AXs were commonly added to wheat flour during dough preparation. It was observed that the addition of water soluble AXs isolated either from rye or from wheat, as well as water insoluble AXs isolated from wheat cause the increase in water absorption, development time and mixing time of wheat dough. The extent of these properties depends on structural features and molar mass of applied AX fractions (Biliaderis et al. 1995; Denli and Ercan 2001; Michniewicz et al. 1992). Both soluble and insoluble AXs exhibit similar water absorption, being higher for those derived from rye than from wheat (Kuhn and Grosch 1989).
Depending on the extraction technique water extractable arabinoxylan (WEAX) and alkali extractable arabinoxylan (AEAX) fractions include more or less amount of AXs with closely interacting components such as protein, starch oligomers, β-glucan, galactan and phenolic substances (Delcour et al. 1999; Dervilly-Pinel et al. 2001; Fengler and Marquardt 1988; Gruppen et al. 1993; Hartmann et al. 2005; Mansberger et al. 2014; Migliori and Gabriele 2010; Schooneveld-Bergmans et al. 1999). The effect of the associated components in the AX fractions could be contrary (Buksa et al. 2012; Hartmann et al. 2005; Mansberger et al. 2014). As an example the application of proteolytic enzymes increases the purity, but at the same time results in hydrolysis of peptides chemically bound to AXs, leading to a decrease in molar mass of AXs compared to those naturally present in the grain.
WEAX were characterized by the degree of arabinose substitution on xylose units (A/X ratio), and for extracted from wheat values of 0.5–0.6 are typical (Courtin and Delcour 2002; Migliori and Gabriele 2010). WEAX extracted from rye, however, have higher or lower A/X ratios, depending on the material, the flour extraction rate, and the applied extraction procedure (Vinkx and Delcour 1996); predominantly the A/X ratio in rye seems to be slightly higher. Rye WEAXs are characterized by a higher amount of mono substituted xylose units in the main chain of AX compared to wheat WEAX (Migliori and Gabriele 2010). In the case of AEAX fractions the A/X ratio is highly dependent on the applied method of extraction (Bushuk 2001; Nilsson et al. 1996).
The number of ferulic acid (FA) molecules linked to wheat or rye AX depends on the location of the biopolymer in the grain (Migliori and Gabriele 2010) and is favored depending on isolation techniques. In alkali extracted AXs, the FA content is very low because of hydrolyzing ester linkages during the alkali treatment (Verwimp et al. 2007).
The molar mass of AX fractions is also dependent on their sources and their extraction techniques (Biliaderis et al. 1995; Buksa et al. 2012; Vinkx and Delcour 1996). HPSEC data of WEAX fraction from wheat and rye (presented by Vinkx et al. 1993) indicate a molar mass in the range of 2.0–3.0 × 105 g/mol. The molar mass of rye WEAX is the highest in this range. The AEAX extracted from wheat and rye showed higher molar mass and dispersity in relation to WEAX (Migliori and Gabriele 2010).
To prove the effectiveness of AX application in the bread making process, it will be useful to record a quality profile from wheat fractions compared to rye fractions under standardized conditions. In this work water extractable and alkali extractable arabinoxylan containing fractions (WEAXs and AEAXs) from wheat- and rye wholemeal extracted in standardized form were characterized (composition, structural features and molecular dimension) by a combination of chemical, physicochemical, enzymatic and chromatographic techniques.
Material and methods
Material
Two wholemeal samples obtained from grain of rye, variety Amilo (Plant Breeding Company Danko, Poland) and wheat, variety Sakwa (Plant Breeding Company HR Strzelce Sp. z o.o., Poland), harvested in the same year (under the same climatic and weather conditions and optimal, controlled fertilization), milled in laboratory mill Lab Mill 3100 (Perten, Sweden), were used as raw material in this work.
The obtained flours were analyzed by determination of starch, dietary fiber and protein content according to AOAC (2006) Methods 996.11, 991.43 and 950.36, respectively. AX content: total (TAX) and water soluble (WEAX) were determined according to Hashimoto et al. (1987). Shortly, TAX were determined by hydrolysis of 10 mg of the sample in 2 mL of 2 M HCl at 100 °C for 2.5 h, neutralization with 2 mL 2 M Na2CO3, yeast addition (2 mL of 2.5 % solution), incubation (1 h at 37 °C) and centrifugation. WEAX were extracted from 100 mg of the sample with water at 30 °C for 2 h. After centrifugation 1 mL of the supernatant was mixed with 1 mL of 4 M HCl and hydrolysis was performed. The analysis of pentoses was carried out by mixing 1 mL of supernatant (for TAX) or 1 mL of hydrolysate (for WEAX) with 2 mL H2O, 3 mL FeCl3 (0.1 % FeCl3 in HCl) and 0.3 mL 1 % orcinol solution in ethanol and boiling for 30 min. After cooling down, the absorbance at 670 nm was measured. The calibration was done with xylose standards of different concentrations.
Preparation of water extractable (WEAX) and alkali extractable (AEAX) arabinoxylans from wholemeal of wheat and rye
40 g of milled whole meal (wheat and rye) was pretreated by heating at 130 °C for 90 min for inactivation of grain enzymes, especially xylanases. Fat was removed from the flour by hexane extraction. After filtration and drying of wholemeal (40 °C, 24 h) water extraction was performed (flour to water ratio was 1:20) at 50 °C for 24 h. The obtained suspension was centrifuged at 1250 × g for 10 min.
In order to free the WEAX extract from starch the clear supernatant was incubated with (70 kU) α-amylase (from Bacillus licheniformis, Sigma-Aldrich) for 60 min at 85 °C. For inactivation of enzyme and coagulation of soluble proteins the solution was heated 30 min at 95 °C, treated with cellite (20 g/L) and filtered by means of Buchner flask. The clear filtrate was poured into 4 times volume of ethanol/acetone solution (1:1). The precipitate was centrifuged at 1250 × g (10 min) and cleaned by repeating dissolving, precipitating and centrifuging steps 3 times. Additionally two washing steps with pure acetone were done in order to remove water totally from the sample. After the last centrifugation the WEAX precipitate was dried at 50 °C for 2 h.
For the extraction of AEAX the precipitate after centrifugation of WEAX fractions containing mainly starch (white layer) and AX (located above the white layer of starch) was used. The AX layer was collected and suspended in 200 mL of Tris-maleate buffer (50 mM, pH 7.5). This suspension was incubated with 70 U of protease (pronase E from Streptomyces griseus, Sigma-Aldrich) at 40 °C for 6 h. Then the pH was adjusted to 6.5 and the solution was incubated with 18,700 U of α-amylase at 70 °C for 2 h. After adjustment of the pH to 7.5 the solution was treated again with 35 U of protease (pronase E from Streptomyces griseus, Sigma-Aldrich) for 12 h, followed by centrifugation. Supernatant was poured out and the residue extracted with 1 M NaOH (200 mL) at room temperature for 24 h. After centrifugation of this extract the resulting residue was once more extracted with 4 M NaOH (100 mL) at room temperature for 16 h. After centrifugation of 4 M NaOH extract, supernatants were combined. The whole solution was acidified to pH = 5.0. AEAXs present in the acidified supernatant were precipitated by pouring into 4 times volume of ethanol/acetone solution (1:1) and further processed as described above for WEAX extraction.
Monosaccharide analysis of arabinoxylans (WEAX and AEAX)
The monosaccharide composition of AXs was determined by HPLC/RI method after acid hydrolysis according to Buksa et al. (2012). Shortly 25 mg arabinoxylan sample was treated with 2 mL of 2 M TFA (trifluoroacetic acid) at 100 °C for 3 h. After centrifugation (2000×g, 5 min), 1.6 mL of the clear supernatant was mixed with 3.4 mL H2O and neutralized with dry Na2CO3 to pH 7 and 20 μL were injected into an HPLC system, consisting of two columns (SP-G and SP08010, Shodex) and RI detector; the eluent was bi-distillated water with a flow rate of 0.6 mL/min; the separation was done at a column temperature of 70 °C. For calibration, monosaccharide standards (glucose, galactose, arabinose, xylose and mannose) at varying concentrations were applied. The content of monosaccharides after hydrolysis was presented as percentage of the sum of all sugars.
Determination of protein content in the extracted preparations
The protein content in the obtained preparations was determined by Total Protein Reagent Biuret Method (Thermo Scientific) measuring the absorbance of the dissolved samples at 540 nm. Calibration curve was done with bovine serum albumin solutions.
Determination of total starch content
Total starch content in the preparations was determined by AOAC (2006) Method 996.11.
Extraction and analysis of total ferulic acid content in AX preparations
Extraction and analysis of total FA from AX preparations was performed according to Buksa et al. (2012). Shortly 50 mg of arabinoxylan sample was treated with 2 mL 2 M NaOH at ambient temperature for 24 h, followed by centrifugation at 2000 × g for 10 min. From the supernatant, 1.5 mL was mixed with 0.8 mL 4 M HCl (to reach pH 2) and extracted with 1.5 mL ethyl acetate and centrifuged (2000 × g, 10 min). This was repeated 3 times for total extraction of FA. The extracts were combined and filled up with ethyl acetate to 5 mL. 1 g of dry Na2SO4 was added to remove residual water from the solution. The FA was measured at 320 nm. The calibration curve was obtained from different concentrations of FA in ethyl acetate. The content of FA was calculated as percentage of dry mass of the preparation and as percentage of AX present in preparation.
Determination of molecular mass distribution of AXs using SEC/RI combined with fraction analysis
20 mg of AX were dissolved in 2 mL of SEC eluent (50 mM NaCl with sodium azide, 50 mg/L) in a reaction glass-tube with screw cap for 15 h at 70 °C using magnetic stirring, followed by short boiling at 100 °C for 30 s and centrifugation at 3500 × g for 10 min. 300 μL of supernatant was injected on SEC column system (Fractogel HW40 (10 × 120 mm) as precolumn + Superose6 (10 × 300 mm) + 2 × Fractogel HW40 (10 × 300 mm)), flow rate 0.6 mL/min, RI detection. Fractions of 1 ml were collected in the range of elution volume from 20 to 60 mL.
Hexoses, pentoses and protein were analysed in the collected fractions:
Determination of hexoses: To 0.5 mL of each fraction 1 mL of anthrone reagent (0.2 g of anthrone (Sigma-Aldrich) per 100 mL of 95 % H2SO4 (POCh, Poland)) was added, incubated at 96 °C for 10 min; absorbance of each fraction was measured at 620 nm.
Determination of pentoses: To 0.3 mL of each fraction 0.7 mL of H2O, 50 μL of ferric ammonium citrate solution (10 mg ferric ammonium citrate per 1 ml H2O) and 1 mL of orcinol reagent (0.2 g orcinol (Sigma-Aldrich) in 100 mL of 95 % H2SO4) were added, incubated at 80 °C for 10 min; absorbance of each fraction was measured at 660 nm.
For determination of protein, absorbance of each fraction was measured at 280 nm.
Calibration of the system was performed with SEC standards: Dextran 670 (401,300 g/mol), Dextran 50 (43,500 g/mol), Dextran 10 (1080 g/mol) obtained from Pharmacosmos (Denmark) and glucose (Sigma-Aldrich); 1 mg of each standard was dissolved together in 1 mL eluent. On the basis of RI signal and data obtained by colorimetric measurements of each fraction the molecular mass distribution and average molar mass Mw were calculated using the software program CPCwin (a.h.group, Graz, Austria).
Determination of molecular mass distribution of AX after xylanase treatment of WEAX and AEAX
10 mg of AX was dissolved in 1 ml SEC-eluent. To 0.8 mL of this sample solution 60 μL of endoxylanase solution (45 U of ß-Xylanase M1 from Trichoderma viride (Megazyme)) was added and incubated at 37 °C for 24 h, boiled and centrifuged at 15,000 × g for 5 min. The supernatant was applied on SEC column system and analyzed as described for determination of molecular mass distribution of AX.
Viscosity measurements
75 mg of the AX sample was dissolved in 15 mL distilled water at 50 °C for 16 h and centrifuged (2000 × g, 15 min). The measurements were performed with Ubbelohde capillary viscometer (Schott, Germany) equipped with automatic timer (ViscoClock) in two replications at constant temperature of 30 °C.
Statistical analysis
All analyses were performed at least in two replications. Standard deviations were calculated using Statistica 8.0 (StatSoft inc., USA) software.
Results and discussion
The chemical composition of the applied wholemeal flours for the extraction of the AX fractions delivered the expected results, being characteristic for wheat and rye (Bushuk 2001). The grain of rye variety Amilo is known for the highest content of AXs compared to wheat and other rye varieties (Buksa et al. 2010). The winter wheat variety Sakwa was according to the European classification a bread–type, common wheat (Triticum aestivum) of low cultivation requirements and high stability to stress conditions (COBORU 2009). For both samples, the content of starch was in the same range, but the protein level in wheat flour was distinctly higher than in rye flour. The content of dietary fiber, however, and consequently the content of AXs in the rye sample were extremely higher compared to the wheat sample. Additionally the water solubility of AXs from rye delivered significantly better results, than from wheat, and promised a good source of WEAX for bakery application (Table S1).
For comparable information of the quality profiles of wheat- and rye AX fractions, extraction and purification of WEAXs and AEAXs from the whole meal flours were carried out under the same standardized conditions. The yield of AX fractions from wheat and rye was significantly different as expected. The yield of WEAX fraction from wheat was only 1.7 % and relatively low compared to the yield of WEAX fraction from rye with 3.2 % of wholemeal samples. Alkali extraction of the samples delivered similar results: wheat AEAX was with the yield of 5.0 % distinctly lower compared to 7.9 % of rye AEAX (Table 1). These results reflected explicitly the existent content of AX in the extracted cereal samples and confirm the better availability of AX from rye grain during the extraction step.
Table 1.
Chemical composition of AX fractions
| Wheat | Rye | |||
|---|---|---|---|---|
| WEAX | AEAX | WEAX | AEAX | |
| Yield of AX isolation (%) | 1.7 | 5.0 | 3.2 | 7.9 |
| Chemical composition of AX fractions | ||||
| AX (%) | 84.3 ± 2.4 | 69.4 ± 1.5 | 89.2 ± 2.7 | 79.5 ± 2.9 |
| Starch* (%) | nd | 13.8 ± 0.8 | nd | 6.1 ± 0.2 |
| Protein (%) | 13.0 ± 0.8 | 12.2 ± 0.9 | 8.4 ± 0.7 | 6.1 ± 1.1 |
| Total FA (mg/100gfrac) | 182.3 ± 2.2 | 5.3 ± 0.2 | 170.3 ± 5.2 | 5.2 ± 0.2 |
| Total FA (mg/100gAX) | 308.0 ± 3.7 | 11.7 ± 0.4 | 250.4 ± 7.6 | 9.3 ± 0.3 |
| Relative composition of sugar moieties after acid hydrolysis of AX fractions | ||||
| Glc (%) | 2.5 ± 0.1 | 21.0 ± 1.0 | 1.7 ± 0.1 | 15.3 ± 1.0 |
| Xyl (%) | 58.2 ± 1.0 | 51.6 ± 0.8 | 61.3 ± 1.1 | 55.9 ± 0.9 |
| Gal (%) | 3.2 ± 0.6 | 0.1 ± 0.0 | n.d. | 0.3 ± 0.1 |
| Ara (%) | 35.8 ± 1.7 | 26.5 ± 1.0 | 37.0 ± 1.5 | 26.2 ± 1.0 |
| Man (%) | 0.3 ± 0.0 | 0.8 ± 0.3 | n.d. | 2.3 ± 0.6 |
| A/X | 0.62 ± 0.01 | 0.51 ± 0.01 | 0.60 ± 0.01 | 0.47 ± 0.01 |
*determined by Total Starch assay (Megazyme)
nd not detected
Chemical composition of WEAX and AEAX fractions
For the purification of the WEAX and AEAX fractions, enzymatic treatment by α-amylase and protease was performed. Checking of starch content by iodine staining in the samples was negative. The application of total starch assay, however, delivered for AEAX fractions distinctly allocable results, 13.8 % for wheat and 6.1 % rye, whereas in WEAX fractions starch assay showed negative results. The different results between iodine staining and starch assay were caused by the high branching characteristics of the rest of starch polymers in the AEAX fractions, being not able to deliver iodine staining.
The content of protein in the samples was proved by means of biuret method. WEAX and AEAX fractions of wheat possessed a relatively high level of protein (Table 1). These results support the assumption that protein in wheat grain is very closely linked to AX molecules in a complex form and cannot be destroyed by either thermal or protease treatment. Similar interaction could be found in the samples of rye; however, the concentration of protein in the WEAX and AEAX fractions was distinctly lower (Table 1).
The calculated content of pure AX in the WEAX fractions, with 84.3 % for wheat and with 89.2 % for rye, was in an acceptable range and confirms the high purity of the fractions. In the AEAX fractions, the content of pure AX was lower and delivered for wheat 69.4 % and for rye 79.5 %. In both cases, foreign components such as protein and starch could not be removed totally by enzymatic treatment during purification and remained in the samples. Surprisingly the content of starch components in AEAX from rye was only the half amount of starch components in AEAX from wheat, referring to a better degradability of rye starch by the applied α-amylase during the purification of samples. Moreover, the closely linked protein prevents a better purification of the AX fractions, particularly from wheat samples.
The WEAX fractions possessed a high content of FA (Table 1) with high ester fixation in the samples. The content of FA in the AEAX fractions, however, was very low (Table 1) because of the strong alkali extraction hydrolyzing the ester linkages between FA and AX molecules (Verwimp et al. 2007) and being responsible for the extensive loss of FA in the samples. FA causes cross-linking of AXs, which could be very important during preparation of the dough. Cross-linked AXs provide higher water absorption and could strengthen the bread crumb. Under this aspect the effectiveness of AEAX fractions both from rye and wheat will be limited due to low content of FA.
Relative composition of monosaccharide after acid hydrolysis
The calculated relative compositions of monosaccharides after acid hydrolysis of the WEAX fractions confirm the high content of pure AX in the wheat and rye samples. The WEAX of wheat delivered besides xylose and arabinose additionally 2.5 % glucose, 3.2 % galactose and traces of mannose allowing speculation about a small amount of water soluble heteropolysaccharides in the sample. In WEAX of rye, however, either galactose nor mannose could be detected; only 1.7 % of glucose was found, eventually originated from water soluble ß-glucan occurring in rye grain. The calculated A/X ratio being an indicator for the structural composition of AX molecules was in the same range for wheat (0.62) and rye (0.60) and hence documents definitely the similar structural features of the extracted water soluble AX fractions.
The relative composition of monosaccharides from the AEAX fractions was dominated by a high content of glucose beside xylose and arabinose, being mainly induced by starch glucan, not totally separated in the preparation process. The rest of unassigned glucose could derive from ß-glucan extracted with alkali from cell wall contained to a small part in wholemeal. Likewise, the low percentage of located mannose and galactose being constituents of hetero-polysaccharides may similarly be originated from cell wall components. Generally these AEAX fractions consist of AXs closely fixed to cell wall components possessing a level of arabinose units about 10 % lower. In this case the water solubility of AX, being closely related to the presence of arabinose, is reduced and can only be extracted with strong alkali (Migliori and Gabriele 2010; Vinkx and Delcour 1996). Additionally the alkali treatment destroys ester linkages with FA and delivers a higher yield of AEAX (Migliori and Gabriele 2010). Under this background, the calculated A/X ratio was for wheat 0.51 and rye 0.47. These values were distinctly lower compared to the WEAX fractions and pointed out the different structural features.
Molecular characteristics before and after endoxylanase treatment
For the SEC (Size Exclusion Chromatography) profile analysis of AX fractions an innovative approach was applied. Additionally to the mass detection, the concentration of pentoses, hexoses and protein were additionally measured by means of specific staining reactions in the collected fractions with spectrometric methods. This novel, simultaneous detection of carbohydrates and protein delivered a useful classification of the available components in the SEC fractions. In case of the specific pentose detection, the adjusted SEC profile of pure AXs could be obtained without extensive purification of AX preparations, which could influence the native structure of these polysaccharides. Likewise, the hexose determination reflected the content of starch or other hexose units containing polysaccharides in the fractions.
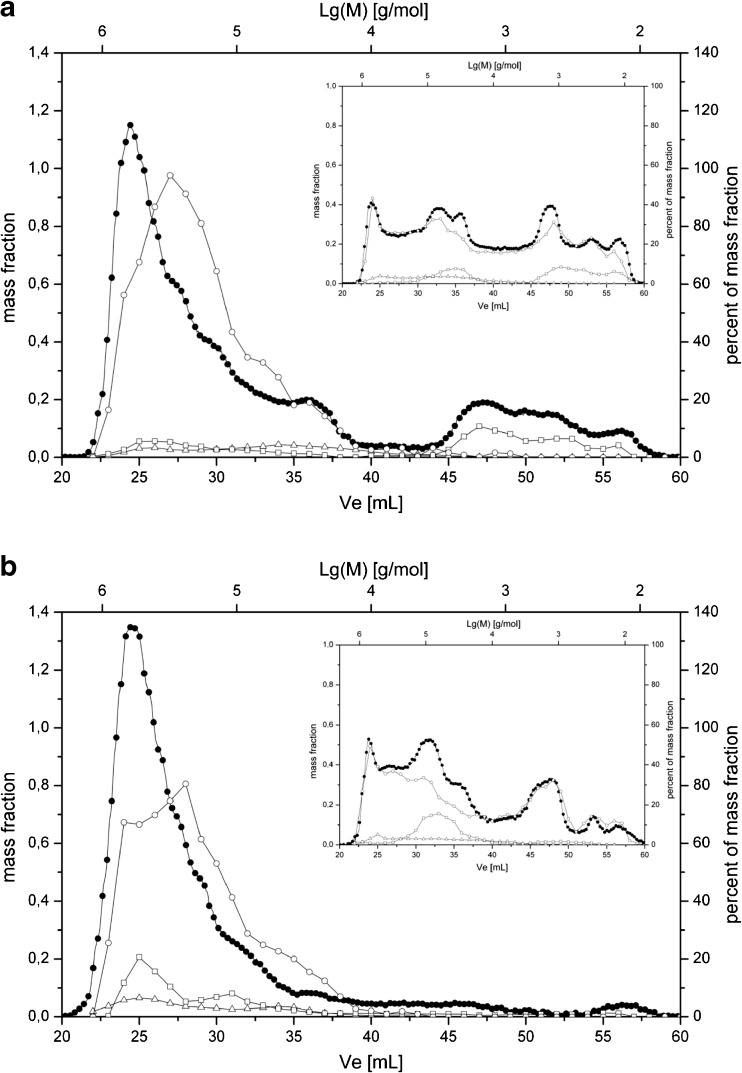
The obtained SEC profiles of WEAX from wheat and rye are shown in Fig. 1a and b. The RI detection delivered approximately the same molar mass distribution for wheat and rye as well as the profile of the pentose detection in the eluted fractions. The part of non-detected components was very low and confirmed the high purity of WEAX fractions. Additionally the profiles of hexoses showed a low level of components supporting the purity of the samples as well.
Fig. 1.
Molecular mass distribution profiles of WEAX from wheat (a) and rye (b); RI signal (−●-), pentoses (−○-), hexoses (−□-), UV at 280 nm (−∆-). Inset: WEAX samples after 24 h xylanase treatment
The UV detection at 280 nm showed a low level of protein interaction in the whole SEC profile confirming the assumption that protein is closely linked to the AX components in all separated fractions.
The molar mass distribution of WEAX fractions was calculated from the SEC profile of pentose detection by means of dextran calibration. The calculated mean values of molar mass distribution were similar for wheat (Mw 176,600 g/mol) and rye (Mw 197,800 g/mol) and delivered a dispersity of 8.1 and 5.2 (Table 2).
Table 2.
Molecular parameters and relative viscosity of wheat and rye AX fractions before and after endoxylanase treatment
| Mean value of molecular mass distribution | Wheat | Rye | ||||||
|---|---|---|---|---|---|---|---|---|
| WEAX | AEAX | WEAX | AEAX | |||||
| Xylanase treatment | Xylanase treatment | |||||||
| before1 | after | before1 | after | before1 | after | before1 | after | |
| Mw [g/mol] | 176 600 | 16 760 | 260 980 | 60 050 | 197 800 | 19 670 | 294 620 | 73 490 |
| Mn [g/mol] | 21 940 | 2 400 | 54 580 | 2 920 | 37 920 | 2 010 | 76 860 | 3 220 |
| Ð (Mw/Mn) | 8.1 | 7.0 | 4.8 | 20.6 | 5.2 | 9.8 | 3.8 | 22.8 |
| Viscosity measurement | ||||||||
| Relative viscosity (ηr) | 1.96 ± 0.01 | 2.04 ± 0.03 | 2.18 ± 0.02 | 2.59 ± 0.01 | ||||
Ð dispersity
1low molecular components not included at the calculation
For detailed information about the structural characteristics of WEAX fractions endoxylanase treatment was performed in combination with SEC profile analysis (Fig. 1a and b, insets). In Table 2 the calculated mean values of the molecular mass distribution are included. Both wheat and rye WEAX show a drastical changing of SEC profiles towards low molecular range; however, parts of high molecular components in the rye fraction could not be degraded. The maximum peak of the broad molar mass distributions was between 40 and 50 elution volume (peak maximum Mw 2100 g/mol for wheat and 2000 g/mol for rye) and verifies the identical degradation of AX molecules from wheat and rye. In the same way, the calculated mean values of molecular mass distribution decreased extremely to values under 20,000 g/mol. The results confirm the similar structural features in both WEAX fractions and support the assumption that the applied endoxylanase splits WEAX only in regions of enough xylose units without arabinose units in the side chain.
According to the data presented in the literature the addition of rye WEAX proved to be more beneficial than the use of wheat-derived preparations (Denli and Ercan 2001; Michniewicz et al. 1992). On the basis of the data presented in this work it could be concluded that the subtle differences in molar mass and structural features of WEAX fractions extracted from rye and wheat will provide slightly better effect of rye WEAX in breadmaking. It is also worth to mention that isolation of WEAX from rye grain is much efficient compared to wheat. The alkali extracted AEAX fractions contain a high level of glucan components first of all due to not totally removed starch components by the applied purification steps. The starch molecules possess a high branching structure and can only be detected with a starch assay kit or in the SEC by analyzing the hexose profile. The SEC profiles for wheat and rye AEAX (Fig. 2a and b) document the total mass distributions (RI-detection) of molecules in comparison to the level of pentoses and hexoses in the eluted fractions.
Fig. 2.
Molecular mass distribution profiles of AEAX from wheat (a) and rye (b); RI signal (−●-), pentoses (−○-), hexoses (−□-), UV at 280 nm (−∆-). Inset: AEAX samples after 24 h xylanase treatment
The hexose detection from the wheat AEAX fraction recorded hexoses containing polymers in a high and a low molecular range of SEC. Both peaks reflected mainly starch components being included in the sample. Preferentially the disintegrated, mostly branched starch molecules were found in the low molecular range of SEC separation. The high molecular range of the SEC separation was related to polymer components being originated from high molecular branched starch, ß-glucan or - in a lower amount - from further heteropolysaccharides containing hexoses. The hexose detection of the rye AEAX fraction showed a double peak in the high molecular range of SEC separation. These hexoses containing components were generated from the existing complex branched starch polymers and ß-glucan combined with heteropolysaccharides being extracted by the strong alkali treatment.
The colorimetric pentose detection of the eluted fractions delivered a distinctly lower level of mass profile for both AEAX fractions in comparison to RI detection. These results underline the less purity of the AEAX fractions and recommend the profiles of pentose detection for the analysis of molar mass distribution. In both cases of wheat and rye, AEAX fractions delivered pentose profiles only in a high molecular range of SEC separation and reflected accurately the molar mass distribution of AX molecules. The mean values were almost identical for wheat (Mw 260,980 g/mol) and for rye (Mw 294,620 g/mol) and possessed a dispersity coefficient of 5.2 and 3.8 (Table 2). Generally the molar mass distribution of AEAX from wheat and rye delivered significantly higher values compared to WEAX and supported the assumption that strong alkali extraction improves solving of the high molecular AX components.
For checking the structural features of AX molecules in the AEAX fractions, endoxylanase treatment was likewise applied in combination with SEC profile analysis. The obtained SEC profiles are shown in the insets in Fig. 2a and b, and the resulting mean values are included in Table 2. The RI detection delivered broad mass distributions over the whole range of separation. The hexose detection showed two peaks in a high and a low molecular range for wheat and only one peak in the high molecular range for rye, similarly to the untreated samples. In both samples, however, the high molecular peak was eluted considerably later because of the releasing of hexose containing molecules from the AX matrix by enzymatic treatment. Likewise, the profiles of pentose detection showed broad mass distributions reduced by the part of hexose containing molecules and were adequate for molar mass analysis of the AX fractions. Both wheat and rye AEAX fractions possessed an acceptable part of AX in the low molecular range with a maximum in approx. 48 mL of elution. These results were similar to the disintegrated molecules of WEAX fraction being composed of regions with high level of unbranched xylose units, convenient for the attack of endoxylananase. Totally different to AX from WEAX the high molar mass components of wheat and rye AEAX fractions were partly resistant against endoxylanase degradation of molecules, probably because of the highly substituted structure of high molecular AX molecules with arabinose units in the side chain. The resulting mean values for enzymatically disintegrated molecules of AEAX confirm the similar action of endoxylanase on wheat and rye fractions with a high dispersity coefficient of 20.6 and 22.8 for the samples.
In the opinion of many authors, water insoluble AXs either have no impact or a detrimental one on breadmaking properties (Krishnarau and Hoseney 1994) and its only beneficial effect is increasing the water absorption of the flour. This property of water insoluble AXs is higher compared to WEAX and rather for those derived from rye than from wheat (Kuhn and Grosch 1989). As it was shown in this work the structural features of AXs (with high degree of rye AEAX), and mainly their high molar mass might be an explanation for higher water absorption of the flour after addition of water insoluble AXs from rye compared to those from wheat (Izydorczyk and Biliaderis 1995).
Viscosity measurement of WEAX and AEAX fractions
From a practical point of view, the relation of molecular properties of polysaccharides to the viscosity of its water solutions is an important issue. Therefore, the relative viscosity of all four obtained fractions was determined (Table 2). The results show that the values of relative viscosities were positively correlated with the molar mass of arabinoxylan fractions, and the viscosities of rye AX samples were slightly higher than the respective wheat AX samples. The viscosities were also correlated with the values of molar mass from AX fractions after endoxylanase treatment and allow the assumption that observed differences in viscosities could be caused by structural differences in the examined AX fractions.
Conclusions
The yield of WEAX fraction from rye was two times as high as from wheat. Both samples of WEAX possessed a high purity with an almost similar chemical composition and molar mass distribution. The A/X ratio of 0.62 (wheat) and 0.60 (rye) as well as the effect of endoxylanase treatment documented the relatively uniform structural composition of xylose and arabinose units in the AX molecules of both samples. The almost identical quality profiles of WEAX fractions establish both substances for an equivalent application in bread making process.
The AEAX fractions deliver quality features different from the WEAX fractions. The yield of AEAX fractions from rye was clearly higher than from wheat similar to the water extraction. Both samples, however, contained residues of not totally removed starch molecules. The molar mass distribution was in high molecular range for both samples and delivered mean values of Mw close to 300,000 g/mol. The A/X ratio was 0.51 for wheat and 0.47 for rye pointing out a lower content of arabinose units in the structural composition of AEAX compared to WEAX. These structural features were confirmed by the results of endoxylanase treatment. In this case, the AEAX fractions partly resisted enzyme attack due to high branched, high molecular AX molecules. The quality profiles of AEAX fractions from wheat and rye were in acceptable accordance and are principally convenient to be applied in bread making process.
Generally the viscosity of AX solutions was strongly influenced by molecular dimensions and the structure of the samples being demonstrated by the fact that samples being less susceptible to endoxylanase action provided solutions with higher viscosity.
Electronic Supplementary Material
(DOCX 12.1 kb)
References
- AOAC . Official methods of analysis. 18th. Gaithersburg, Maryland: Association of Official Analytical Chemists International; 2006. [Google Scholar]
- Biliaderis CG, Izydorczyk MS, Rattan O. Effect of arabinoxylans on bread-making quality of wheat flours. Food Chem. 1995;5:165–171. doi: 10.1016/0308-8146(95)90783-4. [DOI] [Google Scholar]
- Buksa K, Nowotna A, Praznik W, Gambuś H, Ziobro R, Krawontka J. The role of pentosans and starch in baking of wholemeal rye bread. Food Res Int. 2010;43:2045–2051. doi: 10.1016/j.foodres.2010.06.005. [DOI] [Google Scholar]
- Buksa K, Ziobro R, Nowotna A, Praznik W, Gambuś H. Isolation, modification and characterization of soluble arabinoxylan fractions from rye grain. Eur Food Res Technol. 2012;235:385–395. doi: 10.1007/s00217-012-1765-0. [DOI] [Google Scholar]
- Bushuk W. Rye: production, chemistry, and technology. Second. Paul, Minnesota: AAOCC St; 2001. [Google Scholar]
- COBORU Wyniki porejestrowych doświadczeń odmianowych. Zboża Ozime. 2009;2008(64):17. [Google Scholar]
- Courtin CM, Delcour JA. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci. 2002;35:225–243. doi: 10.1006/jcrs.2001.0433. [DOI] [Google Scholar]
- Delcour JA, Rouseu N, Vanhaesendonck IP. Pilot-scale isolation of water-extractable arabinoxylans from rye. Cereal Chem. 1999;76:1–2. doi: 10.1094/CCHEM.1999.76.1.1. [DOI] [Google Scholar]
- Denli E, Ercan R. Effect of added pentosans isolated from wheat and rye grain on some properties of bread. Eur Food Res Technol. 2001;212:374–376. doi: 10.1007/s002170000281. [DOI] [Google Scholar]
- Dervilly-Pinel G, Rimsten L, Saulnier L, Andersson R, Aman P. Water-extractable arabinoxylan from pearled flours of wheat, barley, Rye and Triticale Evidence for the Presence of Ferulic Acid Dimers and Their Involvement in Gel Formation. J Cereal Sci. 2001;34:207–214. doi: 10.1006/jcrs.2001.0392. [DOI] [Google Scholar]
- Fengler AI, Marquardt RR. Water-soluble pentosans from rye: I. Isolation, partial purification, and characterization. Cereal Chem. 1988;65:291–297. [Google Scholar]
- Girhammar U, Nair BM. Isolation, separation and characterization of water soluble non-starch polysaccharides from wheat and rye. Food Hydrocoll. 1992;6:285–299. doi: 10.1016/S0268-005X(09)80096-9. [DOI] [Google Scholar]
- Gruppen H, Kormelink FJM, Voragen AGJ. Water-unextractable Cell Wall Material from Wheat Flour. 3. A Structural Model for Arabinoxylans. J Cereal Sci. 1993;18:111–128. doi: 10.1006/jcrs.1993.1040. [DOI] [Google Scholar]
- Hartmann G, Piber M, Koehler P. Isolation and chemical characterisation of water-extractable arabinoxylans from wheat and rye during breadmaking. Eur Food Res Technol. 2005;221:487–492. doi: 10.1007/s00217-005-1154-z. [DOI] [Google Scholar]
- Hashimoto S, Shogren MD, Pomeranz Y. Cereal arabinoxylans: their estimation and significance. I arabinoxylans in wheat and milled wheat products. Cereal Chem. 1987;64:30–34. [Google Scholar]
- Izydorczyk MS, Biliaderis CG. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr Polym. 1995;28:33–48. doi: 10.1016/0144-8617(95)00077-1. [DOI] [Google Scholar]
- Krishnarau L, Hoseney RC. Enzymes increase loaf volume of bread supplemented with starch tailings and insoluble pentosans. J Food Sci. 1994;59:1251–1254. doi: 10.1111/j.1365-2621.1994.tb14688.x. [DOI] [Google Scholar]
- Kuhn MC, Grosch W. Baking functionality of reconstituted rye flours having different nonstarchy polysaccharide and starch contents. Cereal Chem. 1989;66:149–154. [Google Scholar]
- Mansberger A, D'Amico S, Novalin S, Schmidt J, Tömösközi S, Berghofer E, Schoenlechner R. Pentosan extraction from rye bran on pilot scale for application in gluten-free products. Food Hydrocoll. 2014;35:606–612. doi: 10.1016/j.foodhyd.2013.08.010. [DOI] [Google Scholar]
- Michniewicz J, Biliaderis CG, Bushuk W. Water-insoluble pentosans of wheat: composition and some physical properties. Cereal Chem. 1990;67:434–439. [Google Scholar]
- Michniewicz J, Biliaderis CG, Bushuk W. Effect of added arabinoxylans on some properties of wheat bread. Food Chem. 1992;43:251–257. doi: 10.1016/0308-8146(92)90208-J. [DOI] [Google Scholar]
- Migliori M, Gabriele D. Effect of pentosan addition on dough rheological properties. Food Res Int. 2010;43:2315–2320. doi: 10.1016/j.foodres.2010.08.008. [DOI] [Google Scholar]
- Nilsson M, Saulnier L, Andersson R, Aman P. Water unextractble polisacharydes from three milling fractions of rye grain. Carbohydr Polym. 1996;30:229–237. doi: 10.1016/S0144-8617(96)00071-9. [DOI] [Google Scholar]
- Schooneveld-Bergmans MEF, van Dijk YM, Beldman G, Voragen AGJ. Physicochemical Characteristics of Wheat Bran Glucuronoarabinoxylans. J Cereal Sci. 1999;29:49–61. doi: 10.1006/jcrs.1998.0220. [DOI] [Google Scholar]
- Verwimp T, Van Craeyveld V, Courtin CM, Delcour JA. Variability in the structure of rye flour alkali-extractable arabinoxylans. J Agric Food Chem. 2007;55:1985–1992. doi: 10.1021/jf0623790. [DOI] [PubMed] [Google Scholar]
- Vinkx CJA, Delcour JA. Rye (secale cereale L.) arabinoxylans: a critical review. J Cereal Sci. 1996;24:1–14. doi: 10.1006/jcrs.1996.0032. [DOI] [Google Scholar]
- Vinkx CJA, Reynaert HR, Grobet PJ, Delcour JA. Physicochemical and functional properties of rye nonstarch polysaccharides. V. Variability in the structure and gelling capacity of water soluble arabinoxylans. Cereal Chem. 1993;70:311–317. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12.1 kb)