Abstract
Effect of 2.0 % ginger oil (GO) and 1.5 % ginger extract (GE) in combination with 10.0 % gum arabic (GA) was evaluated for the postharvest control of anthracnose and maintaining quality of Eksotika II papaya fruit during storage at 12 ± 1 °C and 80–85 % RH. Antifungal compounds present in GO and GE were analyzed using gas chromatography and GO was found to contain α-pinene, 1, 8-cineole and borneol, while only borneol was present in GE due to different extraction methods applied. The highest antifungal activity was shown in 2.0 % GO combined with 10 % GA, which significantly (P < 0.05) inhibited spore germination by 93 %. Based on the physicochemical properties tested, 2.0 % GO combined with 10 % GA significantly delayed the ripening of papaya. These results show that 10.0 % GA combined with 2.0 % GO is an effective postharvest biofungicide for papaya.
Keywords: Colletotrichum gloeosporioides, Edible coatings, Sensory evaluation, Disease incidence, Gas chromatography
Introduction
Papaya (Carica papaya L.) is a tropical fruit in the family Caricaceae and its wide acceptability relates to the high content of vitamins, minerals and phenolic compounds. However, the quantity and export value of papaya exported in 2010 was reduced significantly by 38.9 and 20.0 %, respectively when compared with that in 2005 (FAOSTAT 2010). Postharvest losses resulted from anthracnose disease which is one of the main factors that contribute to the significant decrease in the export quantity of papaya.
The shelf life of papaya is often limited as it is highly perishable and susceptible to various postharvest diseases due to its drastic changes in physicochemical properties. In particular, anthracnose caused by Colletotrichum gloeosporioides is a devastating disease that often results in massive economic losses during the transport of papaya. It has been reported that anthracnose disease can cause approximately 50 % losses in fresh commodities (Zahid et al. 2012).
Conventional postharvest treatments to control anthracnose in papaya are heavily relied on the application of synthetic fungicides such as Prochloraz and Propiconazole (Ong et al. 2013). However, prolonged application of single fungicide on fresh fruits and vegetables may result in the development of resistant fungal strains and reduce the effectiveness of fungicide against the target organisms (Edirisinghe et al. 2014; Ali et al. 2010; Maqbool et al. 2011a). Besides the application of synthetic fungicides, several chemical based washing and sanitizing agents such as chlorine have been widely used to control the growth of microorganisms on fresh commodities. It has been suggested that chlorine based washing may negatively affect the physicochemical properties of fresh commodities. The use of chlorine and synthetic fungicides have been restricted due to their carcinogenicity, long degradation time and high residual toxicity which cause serious impact to human health and environment (Zahid et al. 2015; Maqbool et al. 2010). Hot water treatment at 43–49 °C for 20 min is also one of the conventional postharvest treatments that has been used to control anthracnose disease in papaya (Ong et al. 2013). However, under severe conditions of heat treatment, the physical and chemical changes often result in deterioration of nutrients and organoleptic properties of fresh commodities. Therefore, there is a necessity to develop sustainable approaches to minimize the use of synthetic fungicides and chlorine on papaya.
The potential of essential oils as an alternative disease control for fruit such as banana (Maqbool et al. 2010) has been well-studied. To date, a wide range of essential oils such as thyme, Mexican lime, lemongrass, cinnamon and castor oil, applied on papaya fruit have proven to be effective against C. gloeosporioides (Ali et al. 2015; Maqbool et al. 2011a). Sa-Nguanpuag et al. (2011) showed that ginger oil and ginger extract were able to inhibit a wide range of microorganisms such as Bacillus, Pseudomonas, Salmonella and Fusarium. In vitro, Rozwalka et al. (2008) showed that ginger oil has the potential to control C. gloeosporioides. Tripathi et al. (2008) reported an absolute fungitoxic activity of ginger oil against Botrytis cinerea. However, it should be noted that the high volatility, strong aroma, rapid oxidation of bioactive compounds, and the potential toxicity of the essential oils and plant extracts have hindered their potential application on fresh commodities. Therefore, edible coatings can be used as a carrier to maintain high active molecules on the surface of fruits and control postharvest decay (Aloui et al. 2014).
Based on the research that has been conducted previously, the combination of 0.4 % cinnamon oil and gum arabic has successfully suppressed conidial germination by 83.2 %. However, the results obtained were not as effective as when chitosan was applied alone which resulted in 100 % of conidial germination. Considering the antifungal properties of plant extracts and essential oils of underutilised plants which can be easily obtained in the tropical country such as Malaysia, continuous efforts must be taken to improve the efficacy of natural products against postharvest diseases. Therefore, the objectives of this study were to determine the antifungal properties of GO and GE coatings and to study the physicochemical changes of coated papaya during postharvest storage by quality assays and sensory evaluation.
Materials and methods
Plant materials
Mature green papaya fruit with color index of 2 (Carica papaya L. cv. ‘Eksotika II’) were purchased from Exotic Star (M) Sdn Bhd located in Sungai Chua, Kajang, Selangor State of Malaysia on their day of harvest. Papaya fruit of uniform shape, size, maturity and free from physical injury, insect or pathogen infection were selected for the experiment. Fruit were washed with 0.05 % (v/v) of sodium hypochlorite for 3 min, rinsed with purified water and air-dried at room temperature (25 ± 2 °C).
Isolation of C. gloeosporioides and culture conditions
Symptomatic tissues (1 cm2) from the skin of papaya were surface-sterilized using 10 % (v/v) sodium hypochlorite for 5 min, followed by three washes with purified water and drying on sterile paper. The edges of the surface-sterilized tissues were cut off and the tissues were cultured on Petri dishes containing potato dextrose agar (PDA) and incubated at 25 ± 2 °C. Fungal colonies were re-isolated on fresh PDA dishes to obtain pure cultures once mycelial growth was observed. The 7-day old isolates of the fungus which grew on the PDA were identified based on their morphological and cultural properties.
Determination of ginger antifungal compounds
Antifungal compounds of ginger oil and ginger extract were analyzed using a gas chromatograph (Clarus-500, Perkin–Elmer, USA) equipped with a column (Agilent J&W, DB-5MS column: 30 m in length, 0.25 mm in diameter and 0.25 μm in film thickness). Standard solutions were prepared from 100 μl mixtures of 1,8-cineole (v/v), borneol (w/v) and α-pinene (v/v) (Fluka Analytical, USA). They were then added to 900 μl of 99 % (v/v) ethyl alcohol in auto sampler vials. A range of concentrations of α-pinene, 1,8-cineole and borneol were added to the solvent in order to obtain standard calibration curves. For the samples, 100 μl (v/v) of ginger oil and 100 μl (w/v) of ginger extract were added separately to 900 μl of solvent in autosampler vials.
Concentrations of antifungal compounds were measured according to the method described by Sa-Nguanpuag et al. (2011). Temperature adjustment of the GC column was performed in 3 phases. Initial phase involved temperature increment to 60 °C and was maintained for 3 min. In the second phase, the temperature was raised by 15 °C every minute up to 180 °C, which was then maintained for 5 min. The same temperature increment rate was applied in the third phase, up to 250 °C and maintained for 20 min. The temperature of the injector and detector were adjusted to 250 and 280 °C respectively. Helium was selected as the carrier gas, with an average velocity set at 18 psi. The ionization voltage was set at 70 eV. The peak areas present in ginger oil and ginger extract were compared with those areas from standard solutions of α-pinene, 1,8-cineole and borneol at the same retention time in order to determine the concentration of antifungal compounds. The concentrations were expressed in mg ml−1.
Preparation of gum arabic (GA), ginger oil (GO) and ginger extract (GE)
Gum arabic powder (KB-120, Food Grade) was obtained from Jumbo Trading Co. Ltd. Bangkok, Thailand. GA with a concentration of 10 % (w/v) was used as a base coating and it was prepared by dissolving 100 g of GA powder in 1 L purified water. The solution was stirred at 40 °C for 60 min using a hot plate magnetic stirrer. The solution was then filtered using cheesecloth to remove any un-dissolved impurities. After cooling to 20 °C, 0.1 % (v/v) of Tween-80 was added as an emulsifier. It was then adjusted to pH 5.6 with 1 N NaOH using a digital pH meter. Ginger essential oil and ginger extract powder were purchased from Tropical Bioessence Sdn. Bhd, Malacca State of Malaysia. Ginger oil was ready to use, while ginger extract powder was mixed in distilled water to form ginger extract solution. Based on preliminary study (results not shown), 1.5 % (w/v) of GE and 2.0 % (v/v) GO were prepared after optimization procedures were carried out. There were six treatments in total: 10 % GA, 1.5 % GE, 2.0 % GO, 1.5 % GE + 10 % GA and 2.0 % GO +10 % GA.
In vitro antifungal assay
The antifungal assay of ginger oil and extract was performed based on the inhibition in radial mycelial growth of C. gloeosporioides on PDA medium using “the poison food technique” (Sivakumar et al. 2002). Plugs (5 mm in diameter) cut from a pure culture of C. gloeosporioides were placed in the center of PDA dishes containing the treatments. Control dishes contained PDA only. The Petri dishes were incubated at room temperature (25 ± 2 °C). Radial mycelial growth was assessed every 2 days until the mycelium in control dishes reached the edge of the plate. The efficacy of treatments was assessed by comparing growth (mm) of the fungal mycelium.
In vitro spore germination inhibition test was conducted by spreading a spore suspension (1 × 105 spores ml−1) of C. gloeosporioides on the PDA plates with each treatment. The amount of spores in the suspension was determined with a haemacytometer and was adjusted to the desired concentration by dilution with purified water. Control dishes contained PDA only. After 24 h, the spores were observed for germination under a bright field microscope. The number of germinated spores was counted in 10 microscopic fields of 100 spores in 20 replicated plates and presented as percent inhibition.
In vivo antifungal assay
Washed papayas were immersed in the spore suspension (1 × 105 spore ml−1) of C. gloeosporioides for 2–3 min and air-dried at room temperature. Tween 80 (0.1 %) was added to the spore suspension to obtain better adhesion of spores to the surface of fruits (Ali et al. 2011). The fruits were then dipped in 10 % GA, 1.5 % GE, 2.0 % GO, 1.5 % GE + 10 % GA and 2.0 % GO +10 % GA for 2–3 min. The control fruits were dipped in purified water only. The fruits were again air dried and then packed in cardboard boxes and stored at 12 ± 1 °C (80–85 % RH). The effect of various treatments was evaluated at 7 day intervals for 28 days based on the disease incidence and disease severity of the fruit. Disease incidence was calculated as the percentage of fruits showing anthracnose symptoms out of the total number of fruit in each treatment. Disease severity was scored using the scale 1 = 0 % of fruit surface rotten; 2 = 1–25 %; 3 = 26–50 %; 4 = 51–75 % and 5 = 76–100 % (Sivakumar et al. 2002).
Determination of postharvest quality
Weight loss was determined by weighing papaya at day 0 and at the end of each storage interval (weekly). The total weight loss during each storage interval was calculated as the difference between the initial and final fruit weight and recorded as percentages on a fresh weight basis.
Fruit firmness was assessed on each sampling day using an Instron Universal Testing Machine with a probe diameter size of 8 mm in the compression mode with speed of 20 mm/min and load range of 100 N. Measurements were taken for 12 fruits on each sampling day in each treatment at the stem end, midregion and blossom end of each fruit and the average of three readings was recorded and expressed in Newtons (N).
Peel color was analyzed using the Hunter Lab System, Miniscan XE Plus colorimeter calibrated using black and white tiles with values of X = 79.0, Y = 83.9 and Z = 87.9 before readings were taken during each storage interval. The peel color was expressed in chromaticity values of L*, C* and h°. The L* coordinate represented the lightness of color with values ranging from 0 = black to 100 = white. The coordinate C* = (a*2 + b*2)1/2 indicated the hypotenuse of a right angled with values ranging from 0 = least intense to 60 = most intense. The coordinate h° = angle of tangent−1 b*/a* indicated hue, where 0° = red purple, 90° = yellow, 180° = blue-green and 270° = blue.
SSC (°Brix) was determined using a Palette Digital Refractometer (Model: PR-32α Atago Co., Ltd. Japan). Papaya samples (10 g) were ground and homogenized using a kitchen blender with 40 ml of distilled water, and filtered through a muslin cloth. A droplet of the filtrate was then placed on the prism glass of the refractometer which had been calibrated with distilled water prior to taking readings. The readings were multiplied by the dilution factor to obtain the original SSC (%) of the papaya pulp.
The titratable acidity (TA) of papaya was determined by titration against 0.1 N NaoH (Ranggana 1977). Two drops of 0.1 % phenolphthalein (R & M Chemicals, UK) indicator were added to 5 ml of filtrate and NaOH was titrated to turn the solution pink (pH 8.1).
Sensory evaluation of ripe papaya fruit
At the end of the storage period, sensory evaluation of all fruits for peel color, pulp color, aroma, texture, flavor and overall acceptability was performed using the Hedonic scale, method of Allende et al. (2008) with some modifications. A panel of ten untrained judges (comprising different nationality, gender and age) was asked to score the differences between the samples by allotting numbers from 1 to 6, where 1 represented very poor, 2 poor, 3 slightly poor, 4 fair, 5 good, and 6 excellent.
Statistical analysis
For in vitro studies, 4 replicates were used for each treatment with 5 plates in each replicate. For in vivo and physicochemical studies, each treatment had 3 replicates with 4 fruits in each replicate. The treatments were conducted in a completely randomized design (CRD) throughout the experiment. Data were subjected to analysis of variance (ANOVA) using Genstat Software 15th Edition. Means were compared by Least Significant Difference (LSD) tests at P < 0.05.
Results and discussion
Determination of ginger antifungal compounds
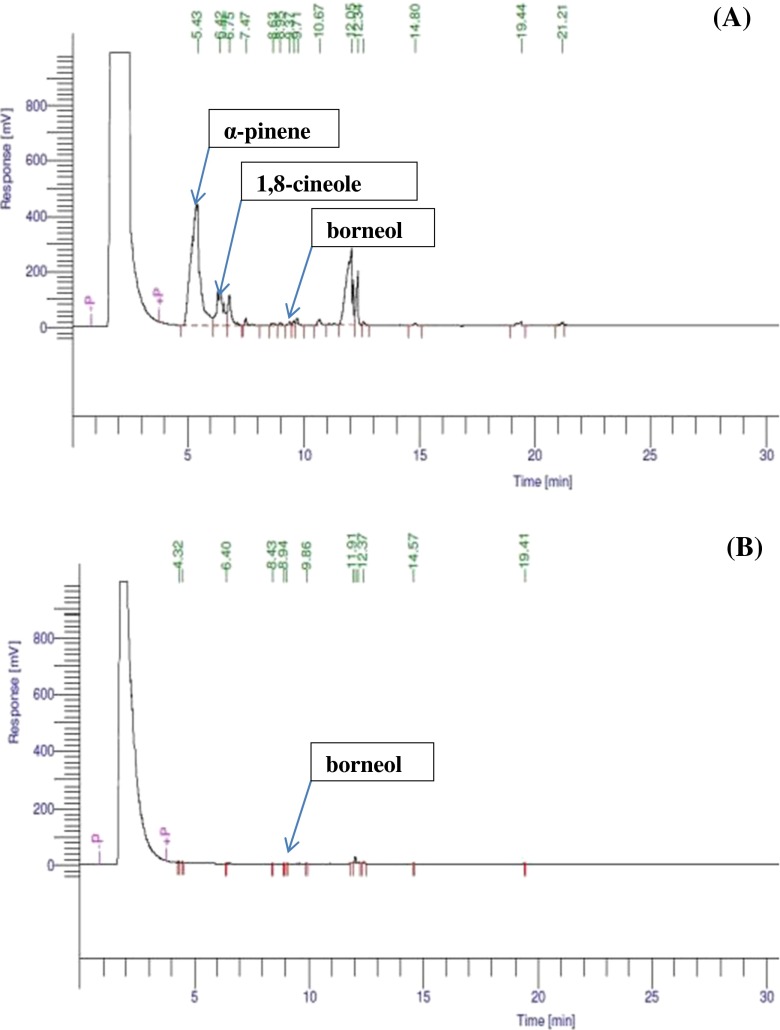
Some antifungal compounds were detected in ginger extract and ginger oil by gas chromatography (Fig. 1a). In ginger oil, α-pinene, 1,8-cineole and borneol were present at 359.89, 125.80 and 40.23 mg/ml with retention times of 5.43, 6.75 and 8.63 min respectively. This agrees with previous reports (Nychas and Skandamis 2003; Sa-Nguanpuag et al. 2011). Terpene compounds, such as α-pinene, borneol, camphene and linalool in ginger, have antimicrobial properties (Nychas and Skandamis 2003).
Fig. 1.
Gas chromatogram of a ginger oil and b ginger extract
The GC analysis of ginger extract revealed less antifungal compounds as compared to ginger oil. A small amount of borneol (0.50 mg/ml) was present (Fig. 1b), which was inconsistent with the study of Sa-Nguanpuag et al. (2011) who determined the major compounds in solvent extracted ginger to be 1,8-cineol and β-phellandrene. These differences in the yield and composition of ginger extract between Sa-Nguanpuag et al. (2011) and the present study could be attributed to the cultivar and its source, solvent used, extraction process, the environmental conditions for growing and harvesting the fruit (Sivasothy et al. 2011; Bautista-Banos et al. 2003).
In vitro antifungal assay of GO, GE and GA
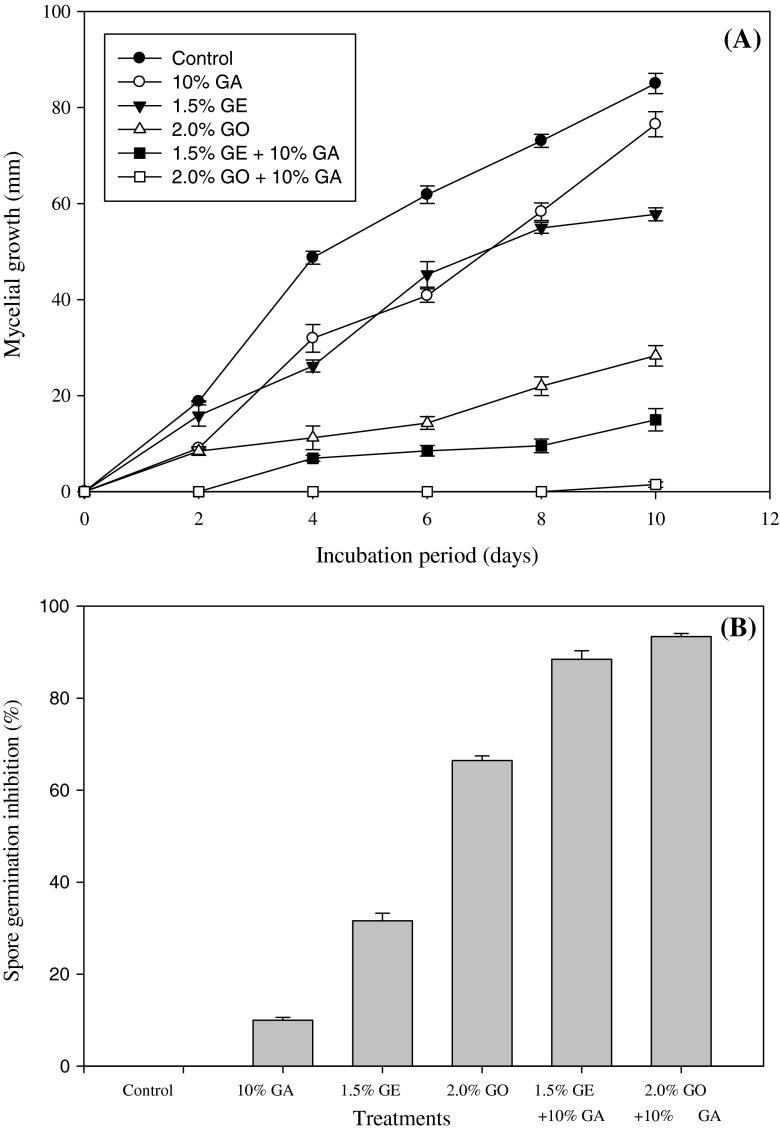
The in vitro study showed that ginger extract and ginger oil had significant antifungal effects against C. gloeosporioides (Fig. 2a). The inhibition of mycelial growth was more conspicuous with ginger oil alone as compared to ginger extract alone. However, a similar growth pattern was observed for both GE and GO combined with GA. The maximum inhibition in mycelial growth was observed with GO combined with GA. There was no inhibition of spore germination of C. gloeosporioides in the control (Fig. 2b) and 10 % inhibition in the GA treatment. Significantly higher inhibition was observed when both GE and GO were combined with GA. It is notable that GO oil exhibited a higher antifungal activity as compared to GE.
Fig. 2.
Effect of different treatments on (A) mycelia growth (mm) of C. gloeosporioides during 10 days of incubation period at 25 ± 2 °C and (B) spore germination inhibition (%) of C. gloeosporioides at 25 ± 2 °C for 24 h. The vertical bars denote the standard error of means for four replicates
Gum arabic (10 %) was selected in this experiment based on the optimum concentrations used for delaying changes in papaya fruit quality (Maqbool et al. 2011b). The same authors reported that GA had no fungicidal effects against C. gloeosporioides, however, mixtures of GA with essential oils were inhibitory (Maqbool et al. 2011a).
In vivo antifungal assay of GO, GE and GA
Gum arabic did not produce any fungicidal effects and disease incidence and severity were similar to that in control fruit (Table 1A and B), reaching a score of 5 and 100 %, respectively, after 28 d of storage. Symptoms of anthracnose appeared on the control and gum arabic treated fruit during the first week of storage. At the end of storage period, most of the fruit were spoiled due to severe disease incidence. Treatments with ginger oil alone and ginger oil with gum arabic delayed the onset of anthracnose during the first week of storage and subsequently the fruit showed less symptoms, with disease severity scores of 2.2 and 2.5 and disease incidence of 20 % and 21 %, respectively, after 28 d of storage.
Table 1.
Effect of different treatments on (A) disease incidence (%) and (B) disease severity (score) of inoculated papaya following 28 d of storage at 12 ± 1 °C and 80–85%RH. Mean values followed by the same significant letters are not significantly different (P > 0.05)
| Treatment | Storage time (days) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |
| (A) Disease incidence (%)* | |||||
| Control | 0.00j | 43.33e-h | 76.67a-c | 86.67ab | 100.00a |
| 10.0 % GA | 0.00j | 26.67 g-i | 70.00b-d | 90.00ab | 100.00a |
| 1.5 % GE | 0.00j | 20.00 h-j | 40.00f-h | 73.33bc | 83.33ab |
| 2.0 % GO | 0.00j | 3.33ij | 20.00 h-j | 46.67d-g | 66.67b-e |
| 1.5%GE + 10.0%GA | 0.00j | 20.00 h-j | 43.33e-h | 70.00b-d | 83.33ab |
| 2.0%GO + 10.0%GA | 0.00j | 3.33ij | 33.33f-h | 53.33c-f | 70.00b-d |
| (B) Disease severity (score)* | |||||
| Control | 1.00 k | 2.37e-i | 3.10c-e | 4.13ab | 4.97a |
| 10.0 % GA | 1.00 k | 2.07f-j | 3.07c-f | 4.20ab | 4.97a |
| 1.5 % GE | 1.00 k | 1.60 h-k | 2.20e-j | 2.63d-g | 3.63b-d |
| 2.0 % GO | 1.00 k | 1.03 k | 1.23jk | 1.80 g-k | 2.17e-j |
| 1.5%GE + 10.0%GA | 1.00 k | 1.37i-k | 2.13e-j | 2.63d-g | 3.70bc |
| 2.0%GO + 10.0%GA | 1.00 k | 1.03 k | 1.60 h-k | 2.00 g-k | 2.47e-h |
In the in vitro experiments the combination of gum arabic with ginger oil or extract gave nearly total inhibition of fungal growth, whereas there was only partial reduction of fungal growth in in vivo experiments. Higher concentrations of essential oil are usually necessary in in vivo experiments due to interactions between phenolic compounds and the food matrix (Feng and Zheng 2007). In addition, the complexity of the food matrix, a nutrient-rich environment, provides an excellent growth medium for fungal replication as well as the repair and regeneration of cellular components (Espitia et al. 2012). Consequently, C. gloeosporioides can be expected to exhibit less sensitivity to ginger oil on the surface of papaya fruit.
Determination of postharvest quality characteristics
Papaya fruit coated with ginger extract, ginger oil and gum arabic lost less weight during storage than the control (p < 0.05) (Table 3A). Permeability of fruit skin is the major factor that causes an increase in water loss during storage (Ali et al. 2014). The basic mechanism of weight loss is caused by vapour pressure differentials at various locations (Yaman and Bayoindirli 2002) and by respiration (Ali et al. 2014). A slower rate of moisture loss and, consequently, a lower weight loss in coated fruit is due to the additional diffusion barrier at stomata (Paull and Chen 1989; Ali et al. 2011), leading to reductions in oxidation reactions and respiration rates (Baldwin et al. 1999).
Table 3.
Effect of treatments on the (A) weight loss, (B) firmness, (C) soluble solid concentration and (D) titratable acidity of papaya following 28 d of storage at 12 ± 1 °C and 80–85%RH. Mean values followed by the same significant letters are not significantly different (P > 0.05)
| Treatment | Storage time (days) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |
| (A) Weight loss (%) | |||||
| Control | 0.00f | 4.59a-f | 9.06a-c | 9.33ab | 11.14a |
| 10.0 % GA | 0.00f | 1.92d-f | 3.55b-f | 6.84a-e | 9.08a-c |
| 1.5 % GE | 0.00f | 2.05d-f | 4.36b-f | 6.25a-f | 7.02a-e |
| 2.0 % GO | 0.00f | 2.91b-f | 3.82b-f | 6.05a-f | 8.01a-d |
| 1.5%GE + 10.0%GA | 0.00f | 1.71d-f | 2.51c-f | 4.14b-f | 5.60a-f |
| 2.0%GO + 10.0%GA | 0.00f | 1.21ef | 2.14d-f | 4.11b-f | 5.38a-f |
| (B) Firmness (N) | |||||
| Control | 104.63a | 17.53d | 17.75d | 16.71d | 20.04d |
| 10.0 % GA | 104.63a | 51.78a-d | 28.17 cd | 24.22 cd | 26.58 cd |
| 1.5 % GE | 104.63a | 63.36 a-d | 51.08a-d | 39.96b-d | 42.75b-d |
| 2.0 % GO | 104.63a | 53.84a-d | 48.78a-d | 43.32b-d | 22.53d |
| 1.5%GE + 10.0%GA | 104.63a | 88.61ab | 55.98a-d | 43.16b-d | 25.71 cd |
| 2.0%GO + 10.0%GA | 104.63a | 102.72a | 79.04a-c | 43.34b-d | 21.83d |
| (C) Soluble solid concentration (%) | |||||
| Control | 6.50b | 10.38ab | 10.67a | 10.29ab | 8.96ab |
| 10.0 % GA | 6.50b | 8.67ab | 10.79a | 10.21ab | 7.96ab |
| 1.5 % GE | 6.50b | 9.92ab | 10.42ab | 8.46ab | 6.88ab |
| 2.0 % GO | 6.50b | 9.58ab | 9.83ab | 8.67ab | 9.29ab |
| 1.5%GE + 10.0%GA | 6.50b | 6.67ab | 9.00ab | 9.33ab | 9.08ab |
| 2.0%GO + 10.0%GA | 6.50b | 7.46ab | 8.21ab | 7.04ab | 10.08ab |
| (D) Titratable acidity (%) | |||||
| Control | 0.050a | 0.034a-c | 0.033a-c | 0.042a-c | 0.040a-c |
| 10.0 % GA | 0.050a | 0.032bc | 0.035a-c | 0.029c | 0.045a-c |
| 1.5 % GE | 0.050a | 0.041a-c | 0.037a-c | 0.040a-c | 0.031bc |
| 2.0 % GO | 0.050a | 0.043a-c | 0.041a-c | 0.042a-c | 0.041a-c |
| 1.5%GE + 10.0%GA | 0.050a | 0.040a-c | 0.050a | 0.042a-c | 0.037a-c |
| 2.0%GO + 10.0%GA | 0.050a | 0.050a | 0.044a-c | 0.04ab | 0.043a-c |
During storage, the papaya fruit became soft in control and coated fruit due to ripening (Table 3B). Gum arabic coated fruit maintained firmness for up to 2 weeks and subsequently showed a similar decrease in firmness as the control. The maximum firmness was maintained in fruit coated with ginger oil and gum arabic (43.3 N), followed by ginger extract with gum arabic (43.1 N) until day 21, after which there were no significant differences between the treatments. Retention of firmness with coating is in agreement with chitosan coated papaya fruit as reported by Bautista-Banos et al. (2003). Martinez-Romero et al. (2006) reported that the higher antifungal activity present in coated fruit is able to reduce infection by covering the cuticle and lenticels in fruit skin, hence minimising respiration and other ripening processes. A low level of oxygen and high level of carbon dioxide tend to limit the activities of hydrolytic enzymes and retain fruit firmness during storage (Salunkhe et al. 1991).
By the end of the storage period, the control fruit had changed from green to yellow with L* = 44.6, chroma =45.1 and hue angle =101.9 (Table 2). The increase in L* and C* values are in agreement with Hernandez et al. (2005) who studied skin color changes of papaya during ripening. Control and ginger oil alone revealed a faster change in color compared to other treatments as shown by L* and C* values on day 21. In contrast, the peel color of fruit coated with ginger extract alone and ginger extract with gum arabic changed more slowly, followed by ginger oil with gum arabic, as indicated by the slower increase in L* and C* values. There was no significant difference in hue angle between the peel of fruit treated with ginger extract plus gum arabic and ginger oil plus gum arabic. Color in papaya fruit is a crucial criterion of quality and consumer acceptability. During the ripening process, alteration in skin color and texture is common. Chlorophyll degradation is often involved in pigmentation changes towards the final stages of papaya fruit ripening (Azevedo et al. 2008) and the biosynthesis of carotenoids (Wills and Widjanarko 1995), resulting in changes of skin color from green to yellowish-orange and red.
Table 2.
Development of (A) L*, (B) C* and (C) h° of peel in Eksotika II papaya coated with different treatments following 28 d of storage at 12 ± 1 °C and 80–85%RH. Mean values followed by the same significant letters are not significantly different (P > 0.05)
| Treatment | Storage time (days) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |
| (A) L* value* | |||||
| Control | 43.5c | 45.9bc | 45.9bc | 55.2a | 44.6c |
| 10.0 % GA | 43.3c | 41.6c | 43.9c | 47.7a-c | 56.0a |
| 1.5 % GE | 40.8c | 41.0c | 42.1c | 42.8 | 46.6bc |
| 2.0 % GO | 41.2c | 45.5bc | 45.9bc | 53.3ab | 53.3ab |
| 1.5%GE + 10.0%GA | 40.8c | 40.3c | 40.8c | 45.2bc | 44.8c |
| 2.0%GO + 10.0%GA | 40.0c | 41.3c | 43.0c | 46.3bc | 46.6bc |
| (B) C* value* | |||||
| Control | 42.3c-f | 42.1ef | 45.6b-f | 55.7a | 45.1b-f |
| 10.0 % GA | 46.9a-f | 47.9a-f | 48.4a-f | 52.0a-c | 54.3ab |
| 1.5 % GE | 43.9c-f | 44.2c-f | 47.0a-f | 47.5a-f | 47.6a-f |
| 2.0 % GO | 39.9f | 44.8b-f | 48.3a-f | 54.0ab | 51.9a-d |
| 1.5%GE + 10.0%GA | 44.8b-f | 42.4c-f | 44.2c-f | 48.7a-f | 48.2a-f |
| 2.0%GO + 10.0%GA | 42.8c-f | 44.8b-f | 46.4a-f | 49.2a-f | 50.5a-e |
| (C) h° value* | |||||
| Control | 113.9a-d | 112.6a-d | 110.7a-d | 101.8f-h | 101.9f-h |
| 10.0 % GA | 113.1a-d | 111.3a-d | 110.9a-d | 107.3c-g | 99.2 h |
| 1.5 % GE | 115.7ab | 114.9a-c | 113.6a-d | 112.9a-d | 99.9gh |
| 2.0 % GO | 116.5a | 112.6a-d | 110.3a-d | 102.1e-h | 100.1gh |
| 1.5%GE + 10.0%GA | 113.8a-d | 114.5a-d | 114.1a-d | 109.7a-e | 109.1a-f |
| 2.0%GO + 10.0%GA | 114.6a-d | 113.5a-d | 112.1a-d | 108.6b-f | 107.1d-g |
The levels of SSC increased for all treatments until day 14 of storage (Table 3C), which is in agreement with results for mature papaya fruit reported by Arriola et al. (1980). This phenomenon may contribute to the breakdown of carbohydrates to simple sugars, and also biosynthesis of carotenoids and organic acids postharvest (Manrique and Lajolo 2004). However, there was a drop in SSC in all treatments starting at day 14, which Moya-Leon et al. (2004) previously considered to be a consequence of fruit senescence. Coated fruit also showed a lower SSC value as compared to control fruit because of the retardation of respiration and metabolic activity, and hence resulted in a slower hydrolysis of carbohydrates to sugars. On day 21, there was a large increment of SSC in fruit treated with ginger oil and gum arabic, which indicated that the fruit can be preserved until day 21 due to the increment in their sweetness.
In control fruit, faster reduction in total acidity (TA) resulted in earlier senescence (Ali et al. 2011). This reduction in the acidity levels during storage was probably due to the increased metabolism of the fruit, resulting in a greater consumption of organic acids in the respiratory process (Espitia et al. 2012). The highest levels of TA were observed in ginger oil with gum arabic which showed a slower reduction in TA throughout the storage period than in the control and gum arabic. Since coated fruit is able to delay ripening by the presence of a semi-permeable film, the lower respiration would delay the consumption of several organic acids including malic and citric acid, which are the primary substrates for respiration (Yaman and Bayoindirli 2002) and the two dominant organic acids in most fruit species (Azevedo et al. 2008). Consequently, a higher TA content was observed in all the coated fruits (Table 3D).
Sensory evaluation of ripe papaya fruit
There were significant differences in flavor, pulp color and overall acceptability of control and coated fruit (Table 4). After 28 days of storage, the sensory characteristics of the papaya fruit treated with ginger extract and gum arabic were far better than those treated with ginger oil and gum arabic. They possessed excellent flavour and highest overall acceptability, with ratings of 4.9 and 4.6 respectively. These coated fruits scored the highest in this study due to the attractive reddish orange pulp and higher sweetness compared to the other treatments. This study has proved that ginger oil and ginger extract do not affect the taste of the Eksotika II papaya as fruit coated by both treatments were well-accepted by panellists.
Table 4.
Effect of treatments on the sensory evaluation of Eksotika II papaya fruit after 28 d of storage at 12 ± 1 °C and 80–85%RH. Mean values followed by the same significant letters are not significantly different (P > 0.05)
| Treatments | Peel colour | Pulp colour | Aroma | Texture | Flavour | Overall acceptability |
|---|---|---|---|---|---|---|
| Control | 3.6a | 2.8e | 3.3a | 2.9b | 2.9b | 3.0b |
| 10.0 % GA | 3.8a | 4.0c | 3.9a | 4.4a | 4.0a | 3.9a |
| 1.5 % GE | 3.1a | 4.7b | 3.4a | 4.2a | 4.1a | 3.8ab |
| 2.0 % GO | 4.0a | 3.8d | 2.8b | 3.3ab | 3.8ab | 3.5ab |
| 1.5 % GE + 10.0 % GA | 3.8a | 4.8a | 3.9a | 4.3a | 4.9a | 4.6a |
| 2.0 % GO +10.0 % GA | 2.9b | 3.8d | 3.5a | 3.7ab | 3.9ab | 4.0a |
Conclusion
Antifungal compounds identified in ginger oil were higher in yield and composition than ginger extract, which indicated higher antifungal activity found in ginger oil. The results further confirmed antifungal properties of ginger oil against C. gloeosporioides. Composite coating with ginger oil and gum arabic was effective in maintaining quality of papaya fruit in term of firmness, peel color, SSC and TA. In conclusion, 2.0 % ginger oil combined with 10 % gum arabic is an effective postharvest biofungicide in controlling anthracnose and maintaining quality of Eksotika II papaya fruit.
Footnotes
Research highlights
• The highest antifungal activity was shown in ginger oil combined with gum arabic
• Fruit coated with ginger edible coatings had less weight loss compared to control
• Ginger extract and gum arabic coated fruit had the highest overall acceptability
• Ginger oil was found to contain α-pinene, 1, 8-cineole and borneol
References
- Ali A, Muhammad MTM, Sijam K, Siddiqui Y. Potential of chitosan coating in delaying the postharvest anthracnose (Colletotrichum gloeosporioides Penz.) of Eksotika II papaya. Int J Food Sci Technol. 2010;45:2134–2140. doi: 10.1111/j.1365-2621.2010.02389.x. [DOI] [Google Scholar]
- Ali A, Muhammad MTM, Sijam K, Siddiqui Y. Effect of chitosan coatings on the physiochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011;124:620–626. doi: 10.1016/j.foodchem.2010.06.085. [DOI] [Google Scholar]
- Ali A, Zahid N, Manickam S, Siddiqui Y, Alderson PG. Double layer coatings: a new technique for maintaining physico-chemical characteristics and antioxidant properties of dragon fruit during storage. Food Bioprocess Tech. 2014;7:2366–2374. doi: 10.1007/s11947-013-1224-3. [DOI] [Google Scholar]
- Ali A, Tan WP, Mustafa MA. Application of lemongrass oil in vapour phase for the effective control of anthracnose of “sekaki” papaya. J Appl Microbiol. 2015;118:1456–1464. doi: 10.1111/jam.12782. [DOI] [PubMed] [Google Scholar]
- Allende A, Selma MV, López-Gálvez F, Villaescusa R, Gil MI. Role of commercial sanitizers and washing systems on epiphytic microorganisms and sensory quality of fresh-cut escarole and lettuce. Postharvest Biol Technol. 2008;49:155–163. doi: 10.1016/j.postharvbio.2007.12.010. [DOI] [Google Scholar]
- Aloui H, Khwaldia K, Licciardello F, Mazzaglia A, Muratore G, Hamdi M, Restuccia C. Efficacy of the combined application of chitosan and locust bean gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int J Food Microbiol. 2014;170:21–28. doi: 10.1016/j.ijfoodmicro.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Arriola MC, Calzada JF, Menchu JF, Rolz C, Garcia R. Tropical and subtropical fruits: composition, properties and uses. Connecticut: Westport; 1980. [Google Scholar]
- Azevedo IG, Oliveira JG, da Silva MG, Pereira T, Corrêa SF, Vargas H, Facanha AR. P-type H + −ATPases activity, membrane integrity, and apoplastic pH during papaya fruit ripening. Postharvest Biol Technol. 2008;48:242–247. doi: 10.1016/j.postharvbio.2007.11.001. [DOI] [Google Scholar]
- Baldwin EA, Burns JK, Kazokas W, Brecht JK, Hagenmaier RD, Bender RJ, Pesis E. Effect of two edible coatings with different permeability characteristics on mango Mangifera indica L. ripening during storage. Postharvest Biol Technol. 1999;17:215–226. doi: 10.1016/S0925-5214(99)00053-8. [DOI] [Google Scholar]
- Bautista-Banos S, Hernández-López M, Bosquez-Molina E, Wilson CL. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003;22:1087–1092. doi: 10.1016/S0261-2194(03)00117-0. [DOI] [Google Scholar]
- Edirisinghe M, Ali A, Maqbool M, Alderson PG. Chitosan controls postharvest anthracnose in bell pepper by activating defense-related enzymes. J Food Sci Technol. 2014;51:4078–4083. doi: 10.1007/s13197-012-0907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espitia PJP, Soares NFF, Botti LCM, Melo NRD, Pereira OL, Silva WAD. Assessment of the efficiency of essential oils in the preservation of postharvest papaya in an antimicrobial packaging system. Braz J Food Technol. 2012;15:307–316. doi: 10.1590/S1981-67232012005000027. [DOI] [Google Scholar]
- Feng W, Zheng X. Essential oils to control Alternaria alternata in vitro and in vivo. Food Control. 2007;18:1126–1130. doi: 10.1016/j.foodcont.2006.05.017. [DOI] [Google Scholar]
- Food and Agriculture Statistics (FAOSTAT) (2010) http://faostat.fao.org/site/342/default.aspx. Accessed 31 June 2013
- Hernandez T, Canales M, Avila JG, Garcia AM, Martinez A, Caballero J, Romo de Vivar A, Lira R. Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf (Verbenaceae) J Ethnopharmacol. 2005;96:551–554. doi: 10.1016/j.jep.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Manrique GD, Lajolo FM. Cell-wall polysaccharide modifications during postharvest ripening of papaya fruit (Carica papaya) Postharvest Biol Technol. 2004;33:11–26. doi: 10.1016/j.postharvbio.2004.01.007. [DOI] [Google Scholar]
- Maqbool M, Ali A, Alderson PG. Effect of cinnamon oil on the incidence of anthracnose and postharvest quality of bananas during storage. Int J Agr Bio. 2010;12:516–520. [Google Scholar]
- Maqbool M, Ali A, Alderson PG, Mohamed MTM, Siddiqui Y, Zahid N. Postharvest application of gum Arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol Technol. 2011;62:71–76. doi: 10.1016/j.postharvbio.2011.04.002. [DOI] [Google Scholar]
- Maqbool M, Ali A, Alderson PG, Zahid N, Siddiqui Y. Effect of a novel edible composite coating based on gum Arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J Agr Food Chem. 2011;59:5474–5482. doi: 10.1021/jf200623m. [DOI] [PubMed] [Google Scholar]
- Martinez-Romero D, Alburquerque N, Valverde JM, Guillen F, Castilo S, Valero D. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: a new edible coating. Postharvest Biol Technol. 2006;39:92–100. doi: 10.1016/j.postharvbio.2005.09.006. [DOI] [Google Scholar]
- Moya-Leon MA, Moya M, Herrera R. Ripening of mountain papaya (Vasconcellea pubescens) and ethylene dependence of some ripening events. Postharvest Biol Technol. 2004;34:211–218. doi: 10.1016/j.postharvbio.2004.05.005. [DOI] [Google Scholar]
- Nychas GJE, Skandamis PN. Antimicrobials from herbs and spices. In: Roller S., editor. Natural antimicrobials for the minimal processing of foods. United Kingdom: Woodhead Publishing; 2003. [Google Scholar]
- Ong MK, Kazi FK, Forney CF, Ali A. Effect of gaseous ozone on papaya anthracnose. Food Bioprocess Tech. 2013;6:2996–3005. doi: 10.1007/s11947-012-1013-4. [DOI] [Google Scholar]
- Paull RE, Chen NJ. Waxing and plastic wraps influence water loss from papaya fruit during storage and ripening. J Am Soc Hortic Sci. 1989;114:937–942. [Google Scholar]
- Ranggana S. Manual analysis of fruit and vegetable products. India: New Delhi; 1977. [Google Scholar]
- Rozwalka LC, Rosa Zaksevskas Da Costa M L, de Mio LLM, Nakashima T. Extracts, decoctions and essential oils of medicinal and aromatic plants in the inhibition of Colleotrichum gloeosporioides and Glomerella cingulata isolates from guava fruits. Ciênc Rural. 2008;38:301–307. doi: 10.1590/S0103-84782008000200001. [DOI] [Google Scholar]
- Salunkhe DK, Boun HR, Reddy NR. Storage processing and nutritional quality of fruits and vegetables. USA: CRC Press; 1991. [Google Scholar]
- Sa-Nguanpuag K, Kanlayanarat S, Srilaong V, Tanprasert K, Techavuthiporn C. Ginger (Zingiber officinale) oil as an antimicrobial agent for minimally processed produce: a case study in shredded green papaya. Int J Agr Biol. 2011;13:895–901. [Google Scholar]
- Sivakumar D, Hewarathgamagae NK, Wijeratnam RSW, Wijesundera RLC. Effect of ammonium carbonate and sodium bicarbonate on anthracnose of papaya. Phytoparasitica. 2002;30:1–7. [Google Scholar]
- Sivasothy Y, Chong WK, Hamid A, Eldeen IM, Sulaiman SF, Awang K. Essential oils of Zingiber officinale var. rubrum theilade and their antibacterial activities. Food Chem. 2011;124:514–517. doi: 10.1016/j.foodchem.2010.06.062. [DOI] [Google Scholar]
- Tripathi P, Dubey NK, Shukla AK. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J Microbiol Biotechnol. 2008;24:39–46. doi: 10.1007/s11274-007-9435-2. [DOI] [Google Scholar]
- Wills RBH, Widjanarko SB. Changes in physiology, composition and sensory characteristics of Australian papaya during ripening. Aust J Exp Agr. 1995;35:1173–1176. doi: 10.1071/EA9951173. [DOI] [Google Scholar]
- Yaman O, Bayoindirli L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Sci Technol. 2002;35:146–150. doi: 10.1006/fstl.2001.0827. [DOI] [Google Scholar]
- Zahid N, Ali A, Manickam S, Siddiqui Y, Maqbool M. Potential of chitosan-loaded nanoemulsions to control different Colletotrichum spp. and maintain quality of tropical fruits during cold storage. J Appl Microbiol. 2012;113:925–933. doi: 10.1111/j.1365-2672.2012.05398.x. [DOI] [PubMed] [Google Scholar]
- Zahid N, Maqbool M, Siddiqui Y, Manickam S, Ali A. Regulation of inducible enzymes and suppression of anthracnose using submicron chitosan dispersions. Sci Hort. 2015;193:381–388. doi: 10.1016/j.scienta.2015.07.014. [DOI] [Google Scholar]