Trachoma, caused by infection with ocular strains of chlamydia, is the leading infectious cause of blindness worldwide. The World Health Organization recommends that in districts where the prevalence of clinically active trachoma exceeds 10% in children aged 1 to 9 years, communities should receive 3 annual mass antibiotic distributions followed by clinical reassessment; any communities with persistent trachoma should continue receiving annual mass antibiotic treatments until the prevalence of clinically active trachoma in children aged 1 to 9 years falls below 5% 1.
Although trachoma treatment decisions are based on the prevalence of clinically active trachoma, it is unclear how quickly the clinical signs of trachoma resolve once infection has been cleared, especially in areas with severe trachoma. We recently performed a series of cluster-randomized clinical trials for trachoma in an area of Ethiopia with hyperendemic trachoma. In these trials, infection was brought to a low level in 24 villages randomized to receive mass azithromycin treatments every 6 months. This provided an opportunity to determine the rate of resolution of the clinical signs of trachoma given little to no chlamydial reinfection.
Methods
Twenty-four randomly selected villages received mass azithromycin treatment every 6 months for 18 months.2,3 In these villages, we monitored the prevalence of ocular chlamydia and clinically active trachoma in all children aged 1 to 5 years 2 months after baseline and then every 6 months after baseline for 24 months. Monitoring preceded mass antibiotic treatment at each of the biannual visits. Ocular chlamydial prevalence was assessed using maximum likelihood estimations from pooled chlamydial polymerase chain reaction (Amplicor; Roche Diagnostics, Branchburg, New Jersey) as described previously.2,3 Clinically active trachoma was defined as follicular trachoma and/or intense inflammatory trachoma according to the World Health Organization's simplified grading system.1 Clinical examiners were trained and validated against gold-standard examiners as described previously 4.
The primary outcome was the resolution rate of clinically active trachoma at the village level. We used a generalized estimating equation to predict the log prevalence of clinically active trachoma during biannual monitoring visits, using time and the log baseline prevalence of clinically active trachoma as fixed effects, village as a random effect, a first-order autoregressive correlation structure, and the Huber-White sandwich estimate of variance. Use of the log-transformed outcome was decided a priori and was supported by plots of the residual vs fitted values. Analyses were performed with Stata version 10.0 statistical software (StataCorp LP, College Station, Texas).
Results
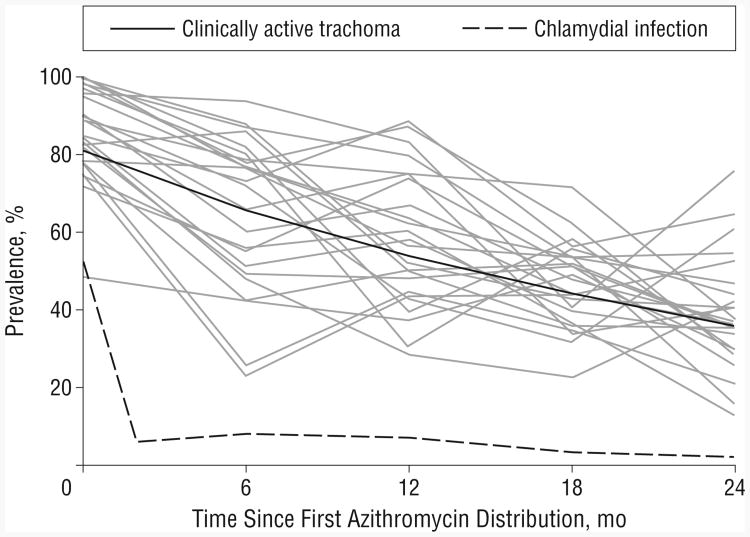
The mean prevalence of ocular chlamydial infection in children before the baseline mass azithromycin treatment was 52.9% (95% confidence interval, 44.2%-61.3%) (Figure).4 Mass azithromycin distribution subsequently occurred at months 0, 6, 12, and 18. The mean prevalence of ocular chlamydia in children during posttreatment biannual monitoring visits was 5.1% (95% confidence interval, 3.6%-6.6%). During these 24 months, the prevalence of clinically active trachoma decreased by 33.3% (95% confidence interval, 28.6%-38.0%) per year as estimated from the generalized estimating equation (Figure).
Figure.
Prevalence of ocular chlamydial infection and clinically active trachoma in 24 Ethiopian villages. At months 0, 6, 12, and 18, mass azithromycin treatments were distributed subsequent to monitoring. Each solid gray line represents an individual village; the solid black line represents the summary of all 24 villages (assessed by generalized estimating equation, assuming the mean baseline prevalence of clinically active trachoma).
Comment
In a hyperendemic area, repeated biannual mass antibiotic treatments reduced the level of ocular chlamydial infection in children to a low level. The prevalence of the clinical signs of active trachoma declined at a much slower rate of 33.3% per year. This rate of resolution is somewhat lower than that reported elsewhere.5,6 However, prior reports have been conducted in fewer villages, with less severe trachoma. In contrast, this study was conducted in many villages in an area with severe trachoma and specifically included the variation among villages in the analysis. Furthermore, our analysis assessed the prevalence of clinically active trachoma in a village as opposed to its presence in an individual. A village-level analysis is most useful for trachoma program managers, who make treatment decisions based on the prevalence of trachoma in a village. Our findings suggest that in areas with hyperendemic trachoma treated through mass distribution of azithromycin, the signs of active trachoma can be expected to resolve slowly, even if antibiotic distributions are quite successful in reducing the prevalence of ocular chlamydia.
Acknowledgments
Funding/Support: This work was supported by grants K23 EY019071 and U10 EY016214 from the National Institutes of Health, the Heed Ophthalmic Foundation, the International Trachoma Initiative, the Bernard Osher Foundation, That Man May See, the Peierls Foundation, the Bodri Foundation, the Harper Inglis Trust, the South Asia Research Fund, and Research to Prevent Blindness.
Footnotes
Financial Disclosure:None reported.
Additional Contributions:The Ethiopian Ministry of Health and many health care professionals, including Tadesse Kebede, Berhanu Fikre, Mifta Shifa, MPH, and Tadesse Birru, helped us organize and implement our fieldwork in Ethiopia.
References
- 1.Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 2.Melese M, Alemayehu W, Lakew T, et al. Comparison of annual and biannual mass antibiotic administration for elimination of infectious trachoma. JAMA. 2008;299(7):778–784. doi: 10.1001/jama.299.7.778. [DOI] [PubMed] [Google Scholar]
- 3.Lakew T, House J, Hong KC, et al. Reduction and return of infectious trachoma in severely affected communities in Ethiopia. PLoS Negl Trop Dis. 2009;3(2):e376. doi: 10.1371/journal.pntd.0000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan JD, Lakew T, Alemayehu W, et al. Clinical activity and polymerase chain reaction evidence of chlamydial infection after repeated mass antibiotic treatments for trachoma. Am J Trop Med Hyg. 2010;82(3):482–487. doi: 10.4269/ajtmh.2010.09-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey RL, Arullendran P, Whittle HC, Mabey DC. Randomised controlled trial of single-dose azithromycin in treatment of trachoma. Lancet. 1993;342(8869):453–456. doi: 10.1016/0140-6736(93)91591-9. [DOI] [PubMed] [Google Scholar]
- 6.Fraser-Hurt N, Bailey RL, Cousens S, Mabey D, Faal H, Mabey DC. Efficacy of oral azithromycin vs topical tetracycline in mass treatment of endemic trachoma. Bull World Health Organ. 2001;79(7):632–640. [PMC free article] [PubMed] [Google Scholar]