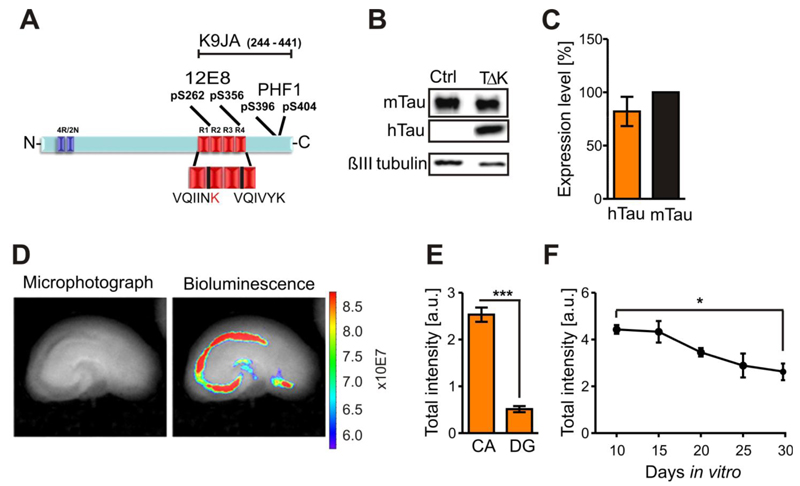
Fig. 1. Human TauRDΔK is highly expressed in area CA1 and CA3 in slice cultures.
(A) TauRDΔK construct is based on the human Tau repeat domain carrying the FTDP-17 mutation ΔK280. Four repeats in the C-terminal half of Tau are highlighted in red (R1-R4). The sequence of the two hexapeptide motifs at beginning of R2 and R3, which promote aggregation by inducing ß-structure, is shown. The FTDP-17 mutation ΔK280 within R2 (marked in red) strongly increase the propensity for ß-structure. Epitopes recognized by phosphorylation-dependent antibodies 12E8 and PHF1 and pan-Tau antibody K9JA are indicated.
(B) Slice homogenates of either control or TauRDΔK cultures were blotted with the Tau antibody K9JA. Endogenous mouse Tau is detected at ~56kDa and TauRDΔKat ~14kDa.
(C) Molar ratio of exogenous TauRDΔKto endogenous mouse Tau is ~0.8:1 (n=6 experiments (6-8 sister slices/group, 6 animals)).
(D) Luciferase activity within TauRDΔK slices (DIV5) were determined by bioluminescence (photons/sec) in area CA and DG (bright field image; left panel). Luciferase activity (right panel) is very high in the CA region and in the remaining cells of the cortex.
(E) Quantification of the luciferase activities in CA and DG region. Area CA1-3 show ~three-fold higher activities (n = 7 slices, 3 animals, Unpaired t-test *** p- value < 0.001).
(F) Luciferase activity monitored over 20 days in vitro. The signal is constant up to 15 DIV but declines thereafter to ~60% of its maximum value at DIV10 (n = 5 experiments, 6 slices/experiment, 3 animals, Unpaired t-test * p- value < 0.05).