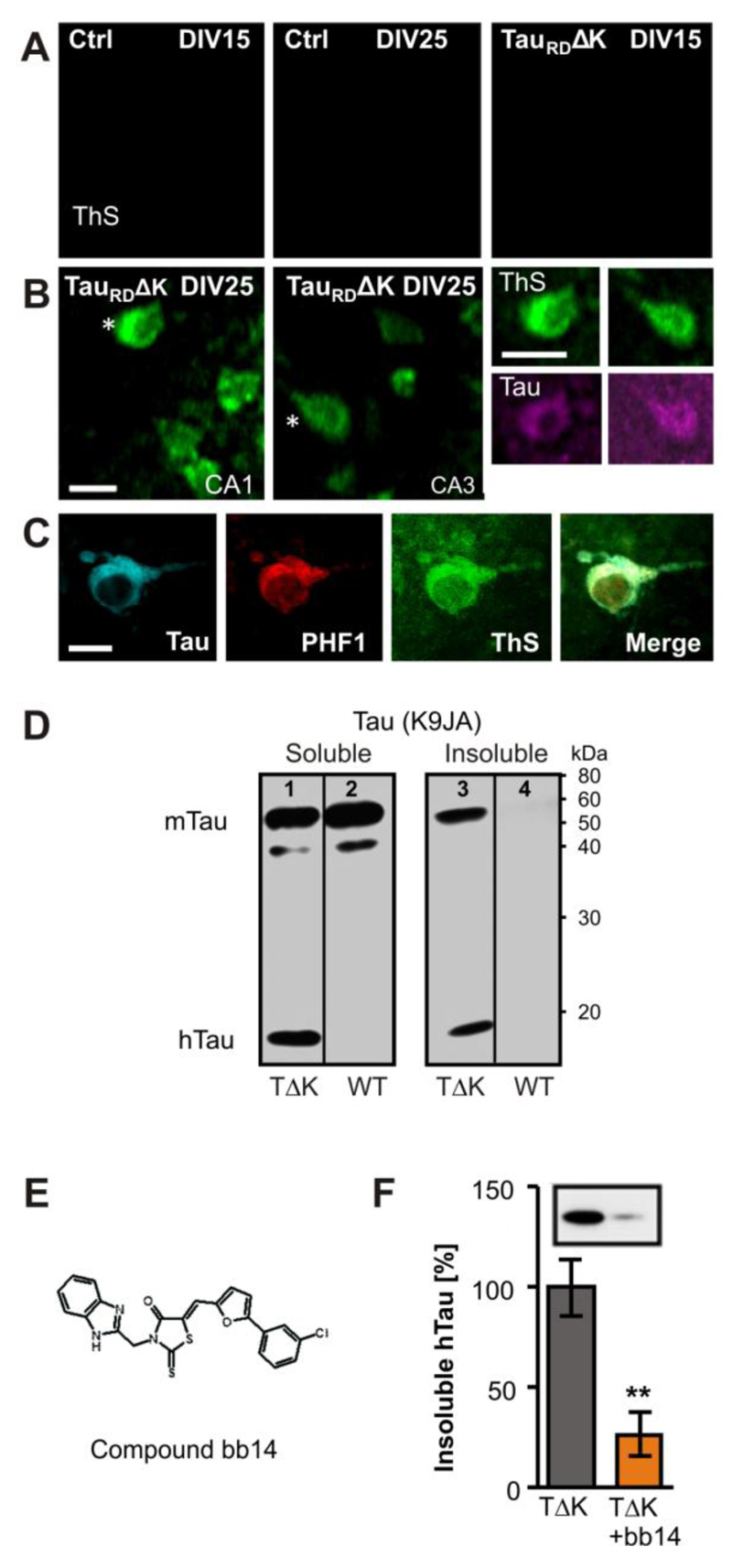
Fig. 2. Expression of TauRDΔK causes Tau aggregation in the CA region.
(A) TauRDΔK or control slices were stained with thioflavine S (ThS) after 15 or 25 days in vitro Control slices showed no ThS signal after 15 or 25 DIV.
(B,C) TauRDΔK slices developed Tau aggregates at DIV 25 but not before. Aggregates appeared inside the somata and could also be stained with K9JA Tau antibodies (asterisks). The co-aggregation of human and mouse Tau was confirmed by antibodies specific for phosphorylated mouse Tau at pS396/pS404 (PHF1).
(D) Western blotting of the soluble (lanes 1, 2) and sarkosyl-insoluble (lanes 3, 4) fraction with K9JA antibodies of DIV25 slices. Human TauRDΔK is detected in homogenates of transgenic slices as a prominent band at Mr ~14kDa in both the soluble (lane 1) and insoluble fractions (lane 3), but not in slices from control mice (lanes 2, 4). In control slices, endogenous mouse Tau occurs only in the soluble fraction. In contrast, in TauRDΔK slices a prominent part of mouse Tau was insoluble, indicating co-aggregation of endogenous and exogenous Tau (lane 3).
(E) Structure of compound bb14, a rhodanine-based tau aggregation inhibitor (Bulic et al, 2007).
(F) Influence of compound bb14 on the aggregation of Tau was investigated by densitometry after western blot analysis with K9JA Tau antibodies of the sarkosyl-insoluble Tau fraction. Compound bb14 (15 µM, treated DIV1-DIV25) reduced the fraction of insoluble TauRDΔK by ~70% (n=4 experiments, unpaired t-test ** p- value < 0.01). Scale bars: A, B, 10µm; C, 5µm.