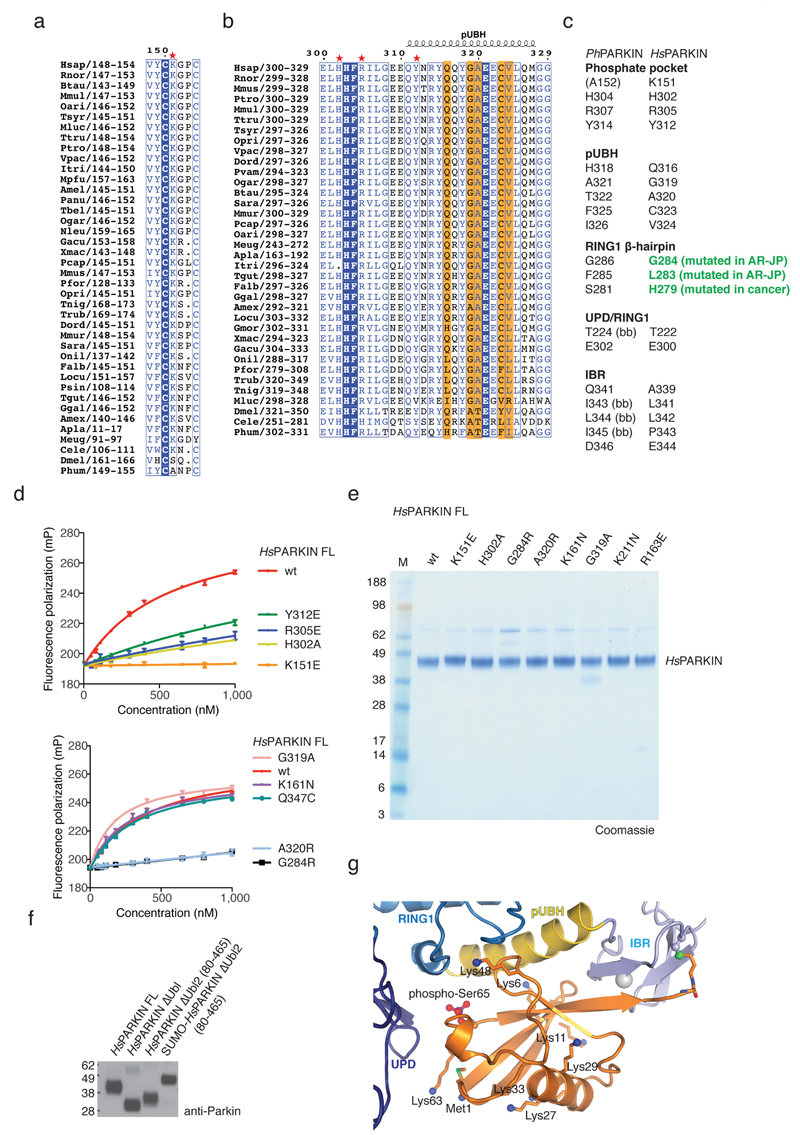
Extended Data Figure 3. Conservation of phosphoUb interacting residues and biochemical analysis.
(a-b) An alignment of PARKIN from species available in Ensembl (www.ensembl.org) was curated by removing sequences with truncations or poorly sequenced regions. The residues involved in phosphoUb binding, comprising (a) the phosphate pocket and (b) the pUBH are shown, and residues contacting phosphoUb are highlighted. (c) Comparison of residues from PhPARKIN and HsPARKIN involved in phosphoUb binding. Highlighted in green are HsPARKIN β-hairpin residues Gly284 (Gly286 in PhPARKIN) which is mutated to Arg in AR-JP. Other β-hairpin mutations, L283P (Phe285 in PhPARKIN) and H279P (Ser281 in PhPARKIN), introduce Pro residues, likely distorting the β-hairpin loop. (bb), backbone interaction. (d) FP assays performed with mutant PARKIN and phosphoUb. Assays from two independent experiments were combined to produce Fig. 2e. Measurements were performed in triplicate with error bars given as standard deviation from the mean. (e) Coomassie stained SDS-PAGE gel showing HsPARKIN proteins used in activity assays. (f) Normalised proteins for PARKIN activity assays in Fig. 3d. (g) Analogous to Fig. 2a, phosphoUb bound to PhPARKIN is shown with Lys residues in stick representation, and free amine groups as blue spheres. Several Lys side chains (Lys29, Lys33, Lys48 and Lys63) were disordered in the electron density maps, suggesting high flexibility and solvent accessibility, and were modelled in their preferred side chain rotamer for illustrative purposes. When bound to PARKIN, each Lys residue can be ubiquitinated, and the C-terminus which is covalently attached to PhPARKIN in the complex structure, is likely more flexible and could also be attached to a more proximal (phospho)Ub in a chain. This indicates that PARKIN could interact with phosphoUb-containing polyUb chains that were reported to be the PINK1 substrate on mitochondria 7,8,11.