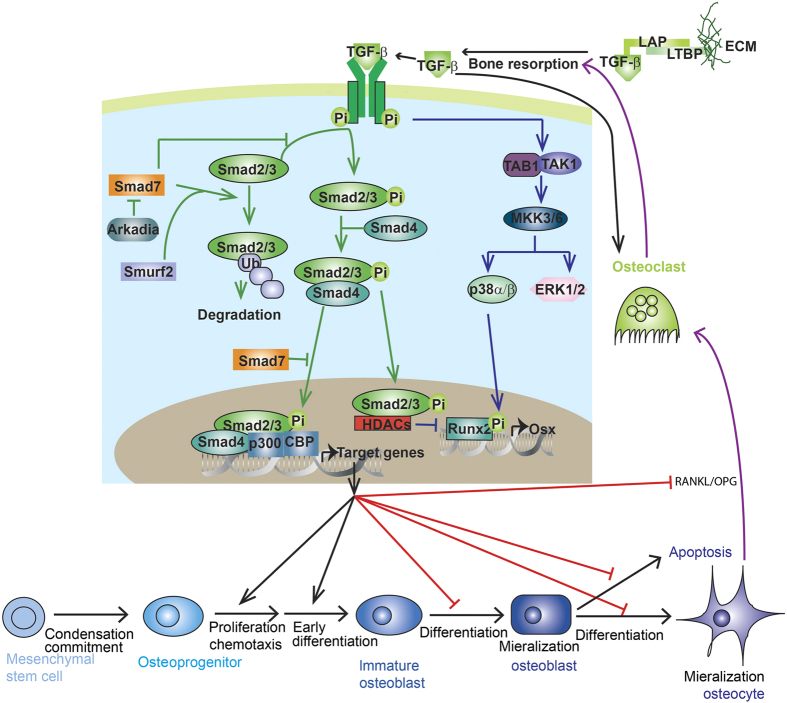
Figure 1.
TGF-β signaling in bone. TGF-β is synthesized as a latent protein stored in the ECM, whose activation depends on osteoclastic bone resorption. Active TGF-βs binds to tetrameric receptor complex comprising two TGF-β types I receptors (TβRI) and two type II receptors (TβRII). TβRII transphosphorylases TβRI to induce Smad-dependent and non-Smad-dependent signaling. In the Smad-dependent signaling, phosphorylated R-Smad (Smad2 or 3) complexes with Smad4 and co-translocates into the nuclei, where they recruit co-factors to regulate target gene expression. In the non-Smad-dependent pathway, phosphorylated TAK1 recruit TAB1 to initiate the MKK-p38 MAPK or MKK–ERK1/2 signaling cascade. Smad7 negatively regulate Smad signaling by preventing R-Smad phosphorylation, targeting R-Smad for ubiquitin–proteasome degradation with Smurf2 and inhibiting R-smad/co-Smad complex nuclei translocation. Arkadia positively regulates Smad signaling by targeting Smad7 for ubiquitin–proteasome degradation. MAPK phosphorylates Runx2 to promote its transcriptional activity while Smad2/3 recruits HDACs to antagonize Runx2 activity. TGF-β–Smad signaling promotes proliferation, chemotaxis, and early differentiation of osteoprogenitor. However, it inhibits osteoblast maturation, mineralization, and transition into osteocyte. It also inhibits osteoclast differentiation by decreasing RANKL/OPG secretion ratio, although it promotes osteoclastogenesis via directly binding with receptors on the osteoclast. TGF-β, transforming growth factor-β.