Abstract
Background
A form of systemic vasculitis, Kawasaki disease (KD) occurs most frequently in children under the age of five years old. Previous studies have found that Prostaglandin E2 (PGE2) correlates with KD, although the related mechanisms are still unknown. CD40L may also be a marker of vasculitis in KD, so this study focuses on PGE2 and CD40L expression in KD.
Materials and Methods
This study consisted of a total of 144 KD patients, whose intravenous immunoglobulin (IVIG)/coronary arterial lesion (CAL) formation resistance was evaluated. PGE2 levels were evaluated in vitro to study the effect of CD40L on CD4+ T lymphocytes.
Results
PGE2 levels significantly increased after IVIG treatment (p<0.05), especially in patients who responded to initial IVIG treatment (p = 0.004) and for patients without CAL formation (p = 0.016). Furthermore, an in vitro study revealed that IVIG acted as a trigger for PGE2 expression in the acute-stage mononuclear cells of KD patients. According to our findings, both IVIG and PGE2 can impede surface CD40L expressions on CD4+ T lymphocytes (p<0.05).
Conclusions
The results of this study are among the first to find that plasma PGE2 is correlated with the prevention of IVIG resistance and CAL formation through CD40L in KD.
Introduction
Kawasaki disease (KD) is a form of systemic vasculitis that was initially described by Tomisaki Kawasaki in 1974[1] and later reported in English. KD occurs throughout the world and generally affects children under the age of five years old. Its most serious complication is coronary artery lesions (CAL)[2], which includes coronary artery fistulas and coronary artery aneurysms [3], and is the primary reason that children develop heart disease [3–5]. Treatment typically involves intravenous immunoglobulin (IVIG) therapy (2 g/kg/dose) combined with high-dose aspirin (80~100 mg/kg/day), which has successfully reduced the prevalence of coronary artery aneurysms in KD patients from 20% to 3–5% [6–8].
Although the cause of KD is not yet known, recent studies have found that endothelial dysfunction (ED) may be a driving force in the progression of KD [9, 10]. PGE2 can both expand the coronary arteries and enhance vascular permeability through four receptor subtypes (EP1, EP2, EP3, and EP4) in a complex way [11], suppress T cell receptor signals, and help resolve inflammation [12]. Some previous studies have already investigated the function of PGE2 in relation to KD [13–15]. Lee et al. (1988) was the first to show that PGE2 plasma levels increased considerably in acute-stage KD and then decreased during the recovery stage in 15 KD patients within their study [13]. Furthermore, another study found that PGE2 could activate β1-integrins through the PGE2 receptor in human coronary arterial endothelial cells [14]. This study also observed that PGE2 often functions via the EP2 receptor in HCAEC and showed that the EP2 antagonist may be able to control the inflammatory response of KD [14]. Meanwhile, prostacyclin analogue has been successfully used to save the extremities of a KD patient with peripheral gangrene [16]. Furthermore, single nucleotide polymorphisms of an ATP-binding cassette, subfamily C, member 4, which is a mediator of prostaglandin efflux, are correlated with KD susceptibility [17]. These findings piqued our interest in the influence of PGE2 on the pathogenesis of KD, and thus this study aims to determine the specific role of PGE2 in both KD’s pathophysiology and its treatment outcomes.
CD40 Ligand (CD40L) is part of the TNF family and is vital to the vascular system’s pathophysiology [18]. In the course of this research, we found both an elevated expression of CD40 ligand (CD40L) on CD4+ helper T cells and platelets in acute-stage KD, as well as a considerably higher expression in KD patients with CAL [19]. Although PGE2 has been proven to inhibit CD40L expression on activated neonatal T cells [20], the clinical importance of both PGE2 and CD40L in KD patients has yet to be properly defined. Furthermore, CD40 and CD40L gene polymorphisms confirmed the association between susceptibility and CAL of KD [21–23]. Researching the plasma PGE2 levels at three different stages of KD and carrying out an in vitro study of primary mononuclear cells from acute-stage KD patients have allowed us to determine the precise role of PEG2 and its relationship with CD40L with regard to the disease outcome of KD patients.
Materials and Method
Patients
A total of 144 KD patients from Kaohsiung Chang Gung Memorial Children’s Hospital in Taiwan from 2007 to 2009 participated in this study. They were all children that met the KD criteria [24, 25] and who were treated with IVIG at the hospital. We also found 50 age-matched febrile control patients that had been admitted to the hospital with a respiratory tract infection, including acute pharyngitis, acute tonsillitis, croup, acute bronchitis, and acute bronchiolitis. Peripheral blood samples were taken at three times: before IVIG treatment (pre-IVIG) and within three days after completing initial IVIG treatment (post-IVIG< 3 days), which served as the acute stage samples, as well as at least three weeks after IVIG treatment, which functioned as the subacute stage samples (post-IVIG> 3 weeks), as described earlier in this paper [26]. CAL formation is defined as a coronary artery with an internal diameter that measured at least 3 mm (4 mm if the patient was older than five years old) or an internal diameter of a segment that was at least 1.5 times that of an adjacent segment, as observed in an echocardiogram [7, 27]. IVIG responsiveness is defined as fever reduction within 48 h of completing IVIG treatment without relapse (temperature >38°C) for at least seven days, as well as obvious improvement or normalization of inflammation [3, 7, 28]. This study was approved by the Chang Gung Memorial Hospital’s Institutional Review Board, and we obtained the written informed consent from the parents or guardians of all the subjects. All of the methods used complied with the approved relevant guidelines.
Plasma PGE2 measurements by enzyme-linked immunoassay (ELISA)
We used the ELISA kit (R&D Systems, Minneapolis, MN) in accordance with the manufacturer's instructions in order to determine the plasma PGE2 levels.
Human mononuclear cell isolation
We freshly isolated peripheral blood mononuclear cells (PBMC) from whole blood using the previously described Ficoll-Paque separation method (Pharmacia, Uppsala, Sweden) [29]. For monocytes isolation, said cells were put in a 100-mm dish (Becton Dickinson, Franklin Lakes, NJ) and allowed to adhere in a 5% CO2 incubator at 37°C for 2 hours. We removed the cells that did not adhere, while the adherent cells were carefully washed at least twice with warm PBS (Biochrom AG) before being harvested. The purity of the resulting cell suspension was then randomly tested using fluorescence-activated cell sorter (FACS) analysis; the sample was deemed pure if it yielded at least 95% monocytes as previous described [30]. Afterward, various concentrations (5, 25, 250, and 2500 mg/dL) of IVIG (7S-IVIG; Gamimmune-N, Bayer, USA) or recombinant PGE2 (0, 10−6, 10−5, and 10-4M, R&D) were incorporated into the PBMC (with monocyte isolation) and PBMC (without monocyte isoloation) for assay of PGE2 and CD40L expression on CD4+ T cells, in a time series of 0.5, 1, 6, 24, and 48 hours, respectively. All tests were separately carried out three times each.
Detecting CD40L expression on CD4+T-Cells
Peripheral venous blood samples were drawn into sterile tubes containing heparin (Becton Dickinson, Heidelberg, Germany). Within 1 hour, 200 μL of whole blood was mixed with 20 μL of appropriate monoclonal antibody conjugates for 30 minutes (4°C in darkness). Anti-CD4 fluorescein isothiocyanate (FITC) (Becton Dickinson) and anti-CD40L phycoerythrin (PE) (Ancell Group, Bayport, MN) were used for staining, while isotype-matched FITC- and PE-conjugated mouse IgG1 (Pharmigen, San Diego, CA) were used as the negative controls. We chose a protein kinase C activator phorbol myristate acetate (PMA, 32 nM) and calcium ionophore (A23187; 1μg/mL) to promote CD40L expression on CD4+ T cells. Following 4 hours of incubation, each sample was collected with a red blood cell lysing buffer (Becton Dickinson), washed two times with cold phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde, and inspected with a FAScan [19]. A total of 104 cells were acquired and inspected using CellQuest software (Becton Dickinson), resulting in cell viability above 95%.
Statistical analysis
All data are presented as mean ± standard error. We analyzed quantitative data with Student’s t-test or, when appropriate, one-way ANOVA; we evaluated any data changes before and after IVIG treatment using the paired sample t-test. A two-sided p-value less than 0.05 is considered statistically significant. All statistical analyses were performed using SPSS version 22.0 for Windows XP (SPSS, Inc., Chicago, USA).
Results
Patient characteristics
Of the 144 KD patients enrolled in this study, 93 (64.6%) were male. The mean age was 1.8±0.15 years old. As for treatment, 130 patients (90.2%) received one dose of IVIG therapy, while the other 14 patients (9.8%) received two IVIG treatments. The echocardiograms revealed that 44 patients (30%) had CAL throughout the entire course of the disease, 11 of which (25%) had received two doses of IVIG therapy (78.6%).
Plasma PGE2 levels in KD patients
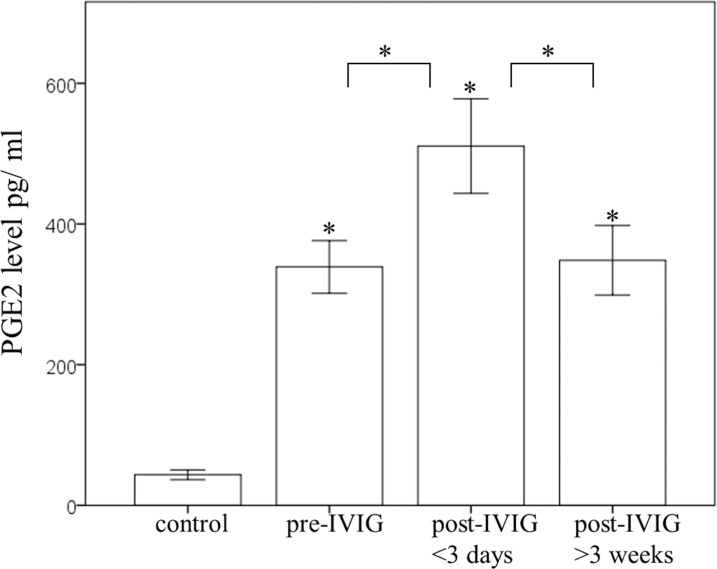
We used ELISA to determine the plasma PGE2 protein levels of the study’s participants. Fig 1 reveals that PGE2 levels were more elevated during all three stages of KD patients than in the control subjects (all p< 0.001). Furthermore, the PGE2 levels significantly increased following IVIG treatment (355 ± 32 pg/ml and 511 ± 72 pg/ml, p = 0.004) and then decreased again during the subacute stage (376 ± 40 pg/ml), which was defined as at least three weeks after KD patients completed treatment with IVIG. We demonstrated that the PGE2 plasma levels did not significantly differ before IVIG treatment and during the subacute stage (p = 0.502).
Fig 1. A comparison of plasma PGE2 levels using ELISA for 15 controls and different stages of Kawasaki disease (KD) (N = 144).
PGE2 levels were higher in all three stages of KD patients than in the control subjects. PGE2 levels significantly increased after patients underwent intravenous immunoglobulin (IVIG) treatment and then decreased during the subacute stage, which was defined as at least three weeks after KD patients completed the IVIG treatment. *indicates p < 0.05 between the control and KD groups. Data are presented as mean ± standard error.
Differences in plasma PGE2 levels related to IVIG treatment response and CAL in KD patients
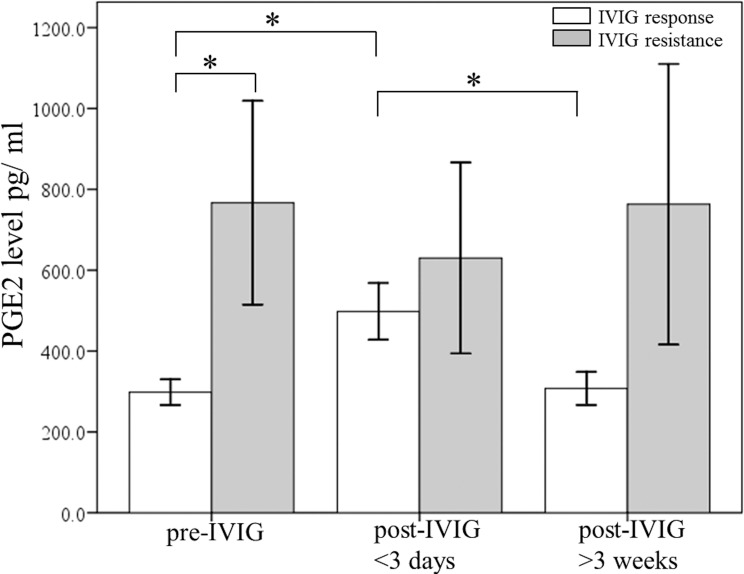
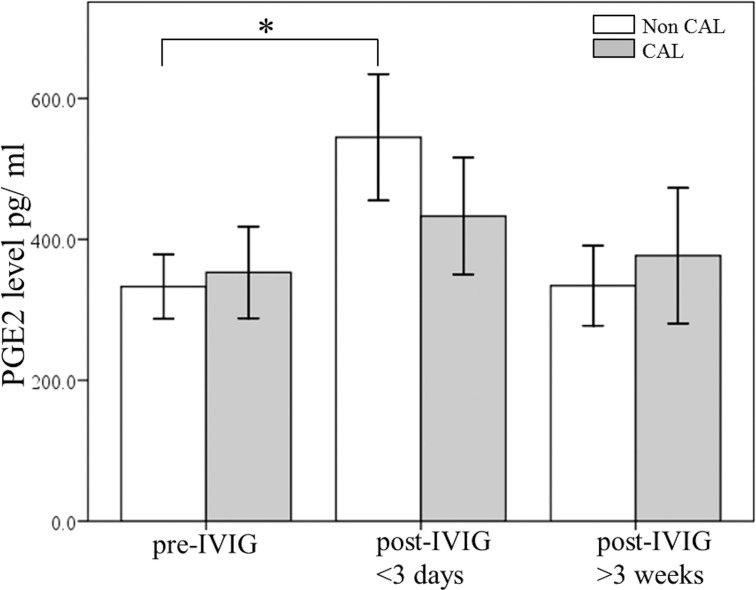
Widespread inflammation is frequently correlated with the occurrence of KD and the subsequent development of CAL, and PGE2 is a key indicator of the anti-inflammatory effect [31, 32]. In order to determine the changes in PGE2 levels after IVIG treatment on CAL formation and its following treatment response, the participating KD patients were divided into two categories: the IVIG resistant group and the IVIG responsive group. Furthermore, Fig 2 shows that the PGE2 levels were higher in the IVIG resistant group than in the IVIG responsive group prior to undergoing IVIG treatment (p = 0.032). We also observed a significant increase in the PGE2 levels of the IVIG responsive group (p = 0.004), but no significant increase of PGE2 levels was found in the IVIG resistance group (p = 0.776). The PGE2 levels of the IVIG-resistant patients were extraordinarily higher during all three time periods and did not differ throughout IVIG treatment. Finally, Fig 3 demonstrates that the PGE2 plasma levels significantly increased following IVIG treatment among the non-CAL group (p = 0.016), while we found no statistical PGE2 increase following IVIG among the CAL group (p = 0.08).
Fig 2. A comparison of plasma PGE2 levels using ELISA between KD patients that responded to IVIG treatment (N = 130) and those that were IVIG resistant (N = 14) prior to receiving IVIG treatment and following IVIG treatment.
*p < 0.05. Data are presented as mean ± standard error.
Fig 3. A comparison of plasma PGE2 levels using ELISA between patients with KD without (n = 100) and with (n = 44) coronary arterial lesions (CAL) prior to receiving IVIG treatment and after IVIG treatment.
*p < 0.05. Data are presented as mean ± standard error.
IVIG regulation of PGE2 expression in PBMC
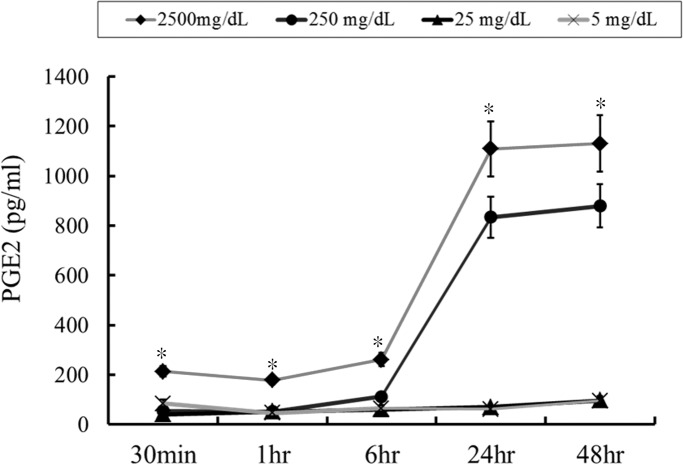
Then PBMC was treated in acute-stage KD patients (N = 4) with various IVIG concentrations in order to evaluate whether IVIG influences PGE2 expression. As predicted, IVIG triggered PGE2 expression considerably in a time-dependent and concentration-dependent manner in PBMC (Fig 4). High dose IVIG (2500mg/dL and 250mg/dL) in 24 and 48 hours significant stimulated PGE2 expression but not found in low dose IVIG (25mg/dL and 5mg/dL). In order to evaluate the importance of monocyte among PBMC in the mechanism of IVIG stimulation PGE2 expression, we removed monocye from PMBC for further study. After removed monocyte from PBMC, we cannot found the significant increase of PGE2 after high dose IVIG stimulation (N = 4, data not shown).
Fig 4. IVIG regulation of PGE2 expression in peripheral blood mononuclear cells.
IVIG significantly triggers PGE2 expression in a time-dependent and dose-dependent manner in peripheral blood mononuclear cells. *p < 0.05 compared to all other groups. Data are presented as mean ± standard error. All tests were separately carried out three times each (N = 4).
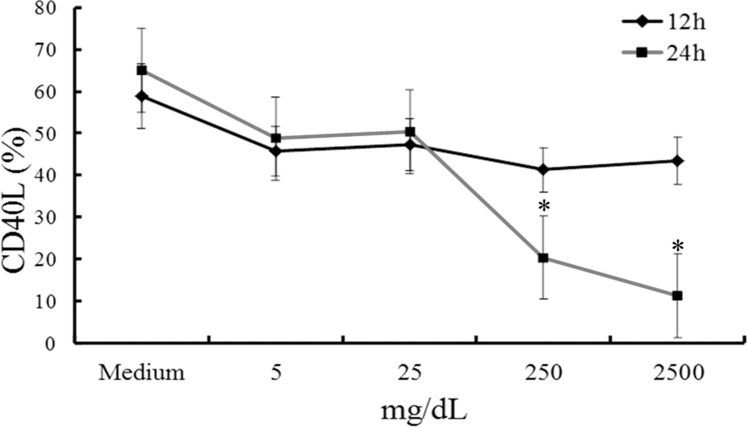
IVIG inhibits CD40L expression on CD4+ T cells
Our research team has already found that an elevated CD40L expression on CD4+ T cells and platelets in acute-stage KD combined with IVIG therapy can successfully reduce CD40L expression in KD patients [19]. Furthermore, we have explored whether IVIG had an affected CD40L expression on CD4+ T cells in vitro. As Fig 5 shows, after an in vitro stimulation by PMA and A23187 for 4 hours, a greater concentration of IVIG was able to significantly inhibit CD40L expression on CD4+ T cells with 24 hours of treatment.
Fig 5. IVIG inhibition of CD40L expression on CD4+ T cells.
Greater concentrations (250 mg/dL and 2500 mg/dL) of IVIG significantly inhibited CD40L expression on CD4+ T cells after 24 hours of treatment.*p < 0.05 compared to the average. Data are presented as mean ± standard error. All tests were separately carried out three times each (N = 4).
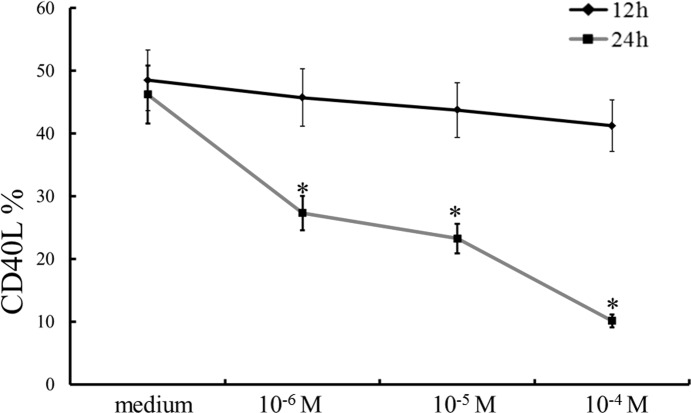
Recombinant PGE2 inhibits CD40L expression on CD4+ T-Cells
This study demonstrated that plasma PGE2 levels did not elevate after IVIG treatment, which correlated with both IVIG resistance and CAL in KD patients. Furthermore, CD40L expression on CD4+ T cells and platelets was related to the manifestation of CAL in KD patients [19]. To determine whether PGE2 can control CD40L expression on CD4+ T cells, various concentrations of recombinant PGE2 were integrated into PBMC after in vitro stimulation by PMA and A23187. Fig 6 demonstrates that PGE2 can inhibit CD40L expression on CD4+ T cells with a dose-dependent method with 24 hours of treatment.
Fig 6. Recombinant PGE2 prevention of CD40L expression on CD4+ T cells.
PGE2 significantly hinders CD40L expression on CD4+T cells in a dose-dependent manner after 24 hours of treatment. *p < 0.05 compared to the average. Data are presented as mean ± standard error. All tests were separately carried out three times each (N = 4).
Discussion
To the best of our knowledge, this is the first study to report that an increase of PGE2 levels following IVIG treatment was observed in patients that responded to IVIG (p = 0.004) and in those without CAL formation (p = 0.016). On the other hand, a significant post-IVIG increase in plasma PGE2 levels was not found in either IVIG non-responders or in patients who had manifested CAL formation. An additional in vitro study confirmed that IVIG could trigger PGE2 expression in PBMC, as well as that both IVIG and PGE2 were capable of blocking CD40L expression on CD4+ T cells in a dose-dependent and time-dependent way.
PGE2 has powerful immunosuppressive characteristics, one of which is blocking the creation of oxygen radical species, leukocyte chemotaxis, proinflammatory cytokines, and chemokines [32, 33]. As a form of vasculitis, KD frequently consists of elevated inflammatory markers and IVIG resistance, which may be related to the development of CAL in KD patients [34–36]. IVIG is well known to exert anti-inflammatory properties. The current study found that PGE2 levels were significantly higher in the participating KD patients upon completing IVIG therapy, as seen in an in vitro study of IVIG stimulation in PBMC from KD patients. Our research team recently found that KD was also related to higher IL-17A and IL-6 levels and that IVIG resistance was related to higher levels of IL-17A [37]. Our findings agree with those of Saha et al., who found that PGE2 negatively normalizes the production of inflammatory cytokines/chemokines and IL-17 in visceral leishmaniasis [38]. Furthermore, PGE2 can enhance Th17 and Th1 cell development [39] and KD was shown to be related to the imbalance of T helper (Th) 17 cells and Treg cells, as well as the down-regulation of the Treg transcription factor FoxP3 expression in KD patients [37, 40]. However, IVIG could promote PGE2 to favor Treg expansion, but can also inhibit Th17 and Th1 cell development [41–44]. In a recent study, our group also demonstrated that IVIG was capable of increasing the expression of Treg-transcription factor FoxP3 in KD patients [37]. In agreement with our findings, the inhibitory activity on toll like receptor agonist-induced IFNα production [45] and the expansion of Treg cells in plasmacytoid dendritic cells or autoimmune patients by IVIG are also required with induction PGE2 levels [46, 47]. A significant increase was also seen in the IP-10 levels of KD patients, which normalized following IVIG treatment [35]. IP-10 was also shown to considerably trigger NK cell cytotoxicity in cases in which immunosuppression by PGE2 took place [48]. In our study, we found that change of PGE2 was a more important predictor of KD outcomes than the actual levels prior to IVIG treatment, as well as an original observation that the lack of increase in PGE2 levels following IVIG treatment correlates with CAL formation and IVIG resistance. By this IVIG brings about immune balance and PGE2 is crucial to immunosuppression in KD patients.
High-dose aspirin is currently the standard treatment for acute-phase KD patients [49]. Inactivation of the cyclooxygenase can hinder the production of prostaglandins. The plasma prostaglandin levels in the high-dose aspirin group were shown to be lower than that in the low-dose aspirin group of KD [50]. Likewise, we discovered that treating KD patients with high-dose aspirin can impair the improvement of the inflammatory markers after IVIG therapy, but the disease outcomes are unaffected [25]. Therefore, high-dose aspirin does not offer any appreciable benefits to acute-phase KD.
The relationship between CD40L and its CD40 receptor is key to controlling inflammatory and immune responses by activating tissue structure cells, such as endothelial cells, smooth muscle epithelial cells, and fibroblasts [51, 52]. We also observed that the CD40L expression on CD4+ T cells was significantly higher in KD patients than in the febrile controls and then significantly decreased three days after completing IVIG therapy [19]. More importantly, CD40L expression on CD4+ T cells and platelets correlated significantly with the manifestation of CAL but CD40L expression on CD8+ T cells or soluble CD40L did not, indicating that CD40L on CD4+ T cells and platelets may be vital to the immunopathogenesis of CAL through interaction with CD40-positive target cells and activation of the immune system and elicit inflammatory reactions, thus resulting in vascular endothelial damage. An in vitro study found that both IVIG and PGE2 could prevent surface CD40L expression on CD4+ T lymphocytes, results that agree with the previous finding that the difference in CD40L expression on CD4+ T lymphocytes after IVIG correlated with CAL in KD. PGE2 and CD40-CD40L signaling have also been shown to have potential effects in patients with atherosclerotic vascular diseases, which helps to explain the increased potential risk for atherosclerosis in KD patients [52–54].
This study’s results are among the first to provide a mechanism to explain the relationships of IVIG/PGE2/CD40L among KD patients. Better comprehension of the fundamental mechanisms of the relationships between IVIG/PGE2/CD40L pathways and long-term coronary arterial remodeling can ultimately result in more effective and innovative treatments for KD patients.
Conclusion
Our findings are foremost in providing proof that a change of plasma PGE2 levels following IVIG treatment was related to IVIG resistance and CAL in KD patients by managing surface CD40L on CD4+ T lymphocytes.
Abbreviations
- CAL
coronary artery lesions
- ELISA
enzyme-linked immunoassay
- IVIG
intravenous immunoglobulin
- KD
Kawasaki disease
- PGE2
Prostaglandin E2
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by funding provided by the following grants: MOST 105-2314-B-182 -050 -MY3, MOST 102-2314-B-182-053-MY3, and MOST 103-2410-H-264-004 from the Ministry of Science and Technology of Taiwan and CMRPG8A0481, CMRPG8E0061, CMRPG8C1082, CMRPG8B0212, CMRPG8D1561, CORPG8F0011, and CMRPG8D0521 from Chang Gung Memorial Hospital in Taiwan. These institutes had no influence over the collection, analysis, or interpretation of the data or on the preparation of this manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):271–6. Epub 1974/09/01. . [PubMed] [Google Scholar]
- 2.Kuo HC, Yang KD, Juo SH, Liang CD, Chen WC, Wang YS, et al. ITPKC single nucleotide polymorphism associated with the Kawasaki disease in a Taiwanese population. PLoS One. 2011;6(4):e17370 Epub 2011/05/03. 10.1371/journal.pone.0017370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang CD, Kuo HC, Yang KD, Wang CL, Ko SF. Coronary artery fistula associated with Kawasaki disease. Am Heart J. 2009;157(3):584–8. Epub 2009/03/03. S0002-8703(08)01043-0 [pii] 10.1016/j.ahj.2008.11.020 . [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364(9433):533–44. Epub 2004/08/11. 10.1016/S0140-6736(04)16814-1 S0140-6736(04)16814-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 5.Kuo HC, Chang WC. Genetic polymorphisms in Kawasaki disease. Acta Pharmacol Sin. 2011;32(10):1193–8. Epub 2011/09/06. 10.1038/aps.2011.93 aps201193 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo HC, Yang KD, Liang CD, Bong CN, Yu HR, Wang L, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol. 2007;18(4):354–9. Epub 2007/06/23. PAI516 [pii] 10.1111/j.1399-3038.2007.00516.x . [DOI] [PubMed] [Google Scholar]
- 7.Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. 2010;99(10):1578–83. Epub 2010/05/25. 10.1111/j.1651-2227.2010.01875.x APA1875 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324(23):1633–9. Epub 1991/06/06. 10.1056/NEJM199106063242305 . [DOI] [PubMed] [Google Scholar]
- 9.Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum Albumin Level Predicts Initial Intravenous Immunoglobulin Treatment Failure in Kawasaki Disease. Acta Paediatr. 2010. Epub 2010/05/25. APA1875 [pii] 10.1111/j.1651-2227.2010.01875.x . [DOI] [PubMed] [Google Scholar]
- 10.Yamakawa R, Ishii M, Sugimura T, Akagi T, Eto G, Iemura M, et al. Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol. 1998;31(5):1074–80. Epub 1998/04/30. S0735-1097(98)00033-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 11.Schror K. The effect of prostaglandins and thromboxane A2 on coronary vessel tone—mechanisms of action and therapeutic implications. European heart journal. 1993;14 Suppl I:34–41. . [PubMed] [Google Scholar]
- 12.Wiemer AJ, Hegde S, Gumperz JE, Huttenlocher A. A live imaging cell motility screen identifies prostaglandin E2 as a T cell stop signal antagonist. Journal of immunology. 2011;187(7):3663–70. 10.4049/jimmunol.1100103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T, Furukawa S, Fukuda Y, Yabuta K, Kato H. Plasma prostaglandin E2 level in Kawasaki disease. Prostaglandins Leukot Essent Fatty Acids. 1988;31(2):53–7. Epub 1988/02/01. . [DOI] [PubMed] [Google Scholar]
- 14.Kajimoto M, Ichiyama T, Ueno Y, Shiraishi M, Hasegawa M, Furukawa S. Enhancement of activated beta1-integrin expression by prostaglandin E2 via EP receptors in isolated human coronary arterial endothelial cells: implication for the treatment of Kawasaki disease. Inflammation research: official journal of the European Histamine Research Society [et al]. 2009;58(4):224–8. 10.1007/s00011-008-8138-y . [DOI] [PubMed] [Google Scholar]
- 15.Sasai K. [Plasma PGE2, TXB2 and 6-keto PGF1 alpha levels in patients with Kawasaki disease]. Arerugi = [Allergy]. 1988;37(9):952–8. . [PubMed] [Google Scholar]
- 16.Dogan OF, Kara A, Devrim I, Tezer H, Besbas N, Ozen S, et al. Peripheral gangrene associated with Kawasaki disease and successful management using prostacycline analogue: a case report. The heart surgery forum. 2007;10(1):E70–2. 10.1532/HSF98.20061151 . [DOI] [PubMed] [Google Scholar]
- 17.Khor CC, Davila S, Shimizu C, Sheng S, Matsubara T, Suzuki Y, et al. Genome-wide linkage and association mapping identify susceptibility alleles in ABCC4 for Kawasaki disease. Journal of medical genetics. 2011;48(7):467–72. 10.1136/jmg.2010.086611 . [DOI] [PubMed] [Google Scholar]
- 18.Hassan GS, Merhi Y, Mourad W. CD40 ligand: a neo-inflammatory molecule in vascular diseases. Immunobiology. 2012;217(5):521–32. 10.1016/j.imbio.2011.03.010 . [DOI] [PubMed] [Google Scholar]
- 19.Wang CL, Wu YT, Liu CA, Lin MW, Lee CJ, Huang LT, et al. Expression of CD40 ligand on CD4+ T-cells and platelets correlated to the coronary artery lesion and disease progress in Kawasaki disease. Pediatrics. 2003;111(2):E140–7. . [DOI] [PubMed] [Google Scholar]
- 20.Splawski JB, Nishioka J, Nishioka Y, Lipsky PE. CD40 ligand is expressed and functional on activated neonatal T cells. Journal of immunology. 1996;156(1):119–27. . [PubMed] [Google Scholar]
- 21.Onouchi Y, Onoue S, Tamari M, Wakui K, Fukushima Y, Yashiro M, et al. CD40 ligand gene and Kawasaki disease. European journal of human genetics: EJHG. 2004;12(12):1062–8. 10.1038/sj.ejhg.5201266 . [DOI] [PubMed] [Google Scholar]
- 22.Kuo HC, Chao MC, Hsu YW, Lin YC, Huang YH, Yu HR, et al. CD40 Gene polymorphisms associated with susceptibility and coronary artery lesions of Kawasaki disease in the Taiwanese population. ScientificWorldJournal. 2012;2012:520865 10.1100/2012/520865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, Hamada H, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012;44(5):517–21. 10.1038/ng.2220 . [DOI] [PubMed] [Google Scholar]
- 24.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–71. Epub 2004/10/27. 110/17/2747 [pii] 10.1161/01.CIR.0000145143.19711.78 . [DOI] [PubMed] [Google Scholar]
- 25.Kuo HC, Lo MH, Hsieh KS, Guo MM, Huang YH. High-Dose Aspirin Is Associated with Anemia and Does Not Confer Benefit to Disease Outcomes in Kawasaki Disease. PLoS One. 2015;10(12):e0144603 10.1371/journal.pone.0144603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiao MM, Huang LT, Chen CJ, Sheen JM, Tain YL, Chen CC, et al. Melatonin in the regulation of liver steatosis following prenatal glucocorticoid exposure. BioMed research international. 2014;2014:942172 10.1155/2014/942172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman ST, De Inocencio J, Hirsch R. Kawasaki disease. Pediatr Clin North Am. 1995;42(5):1205–22. Epub 1995/10/01. . [DOI] [PubMed] [Google Scholar]
- 28.Kuo HC, Yu HR, Juo SH, Yang KD, Wang YS, Liang CD, et al. CASP3 gene single-nucleotide polymorphism (rs72689236) and Kawasaki disease in Taiwanese children. J Hum Genet. 2011;56(2):161–5. Epub 2010/12/17. jhg2010154 [pii] 10.1038/jhg.2010.154 . [DOI] [PubMed] [Google Scholar]
- 29.Weiss G, Murr C, Zoller H, Haun M, Widner B, Ludescher C, et al. Modulation of neopterin formation and tryptophan degradation by Th1- and Th2-derived cytokines in human monocytic cells. Clin Exp Immunol. 1999;116(3):435–40. Epub 1999/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carosella ED, Pascal N, Armand J. Fc fragment from human IgG induces PGE2 secretion. II. Negative regulation in B cell differentiation. Cellular immunology. 1989;121(2):261–8. . [DOI] [PubMed] [Google Scholar]
- 31.Na YR, Jung D, Yoon BR, Lee WW, Seok SH. Endogenous prostaglandin E2 potentiates anti-inflammatory phenotype of macrophage through the CREB-C/EBP-beta cascade. European journal of immunology. 2015;45(9):2661–71. 10.1002/eji.201545471 . [DOI] [PubMed] [Google Scholar]
- 32.Saha A, Biswas A, Srivastav S, Mukherjee M, Das PK, Ukil A. Prostaglandin E2 negatively regulates the production of inflammatory cytokines/chemokines and IL-17 in visceral leishmaniasis. Journal of immunology. 2014;193(5):2330–9. 10.4049/jimmunol.1400399 . [DOI] [PubMed] [Google Scholar]
- 33.Jing H, Yen JH, Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. The Journal of biological chemistry. 2004;279(53):55176–86. 10.1074/jbc.M409816200 . [DOI] [PubMed] [Google Scholar]
- 34.Kim JJ, Hong YM, Yun SW, Han MK, Lee KY, Song MS, et al. Assessment of Risk Factors for Korean Children with Kawasaki Disease. Pediatr Cardiol. 2011. Epub 2011/11/23. 10.1007/s00246-011-0143-1 . [DOI] [PubMed] [Google Scholar]
- 35.Ko TM, Kuo HC, Chang JS, Chen SP, Liu YM, Chen HW, et al. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circulation research. 2015;116(5):876–83. 10.1161/CIRCRESAHA.116.305834 . [DOI] [PubMed] [Google Scholar]
- 36.Kuo HC, Yang YL, Chuang JH, Tiao MM, Yu HR, Huang LT, et al. Inflammation-induced hepcidin is associated with the development of anemia and coronary artery lesions in Kawasaki disease. Journal of clinical immunology. 2012;32(4):746–52. 10.1007/s10875-012-9668-1 . [DOI] [PubMed] [Google Scholar]
- 37.Guo MM, Tseng WN, Ko CH, Pan HM, Hsieh KS, Kuo HC. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy. 2015;70(3):310–8. 10.1111/all.12558 . [DOI] [PubMed] [Google Scholar]
- 38.Shah SV, Rajapurkar MM, Baliga R. The role of catalytic iron in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(10):2329–31. Epub 2011/10/08. 6/10/2329 [pii] 10.2215/CJN.08340811 . [DOI] [PubMed] [Google Scholar]
- 39.Chizzolini C, Brembilla NC. Prostaglandin E2: igniting the fire. Immunology and cell biology. 2009;87(7):510–1. 10.1038/icb.2009.56 . [DOI] [PubMed] [Google Scholar]
- 40.Jia S, Li C, Wang G, Yang J, Zu Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin Exp Immunol. 2010;162(1):131–7. 10.1111/j.1365-2249.2010.04236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maddur MS, Rabin M, Hegde P, Bolgert F, Guy M, Vallat JM, et al. Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain-Barre syndrome patients. Immunologic research. 2014;60(2–3):320–9. 10.1007/s12026-014-8580-6 . [DOI] [PubMed] [Google Scholar]
- 42.Li S, Jin T, Zhang HL, Yu H, Meng F, Concha Quezada H, et al. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barre syndrome and downregulated by IVIg treatments. Mediators of inflammation. 2014;2014:740947 10.1155/2014/740947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maddur MS, Sharma M, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibitory effect of IVIG on IL-17 production by Th17 cells is independent of anti-IL-17 antibodies in the immunoglobulin preparations. J Clin Immunol. 2013;33 Suppl 1:S62–6. 10.1007/s10875-012-9752-6 . [DOI] [PubMed] [Google Scholar]
- 44.Maddur MS, Vani J, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin. The Journal of allergy and clinical immunology. 2011;127(3):823–30 e1-7. 10.1016/j.jaci.2010.12.1102 . [DOI] [PubMed] [Google Scholar]
- 45.Wiedeman AE, Santer DM, Yan W, Miescher S, Kasermann F, Elkon KB. Contrasting mechanisms of interferon-alpha inhibition by intravenous immunoglobulin after induction by immune complexes versus Toll-like receptor agonists. Arthritis Rheum. 2013;65(10):2713–23. 10.1002/art.38082 . [DOI] [PubMed] [Google Scholar]
- 46.Maddur MS, Trinath J, Rabin M, Bolgert F, Guy M, Vallat JM, et al. Intravenous immunoglobulin-mediated expansion of regulatory T cells in autoimmune patients is associated with increased prostaglandin E2 levels in the circulation. Cellular & molecular immunology. 2015;12(5):650–2. 10.1038/cmi.2014.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood. 2013;122(8):1419–27. 10.1182/blood-2012-11-468264 . [DOI] [PubMed] [Google Scholar]
- 48.Muller C, Tufa DM, Chatterjee D, Muhlradt PF, Schmidt RE, Jacobs R. The TLR-2/TLR-6 agonist macrophage-activating lipopeptide-2 augments human NK cell cytotoxicity when PGE2 production by monocytes is inhibited by a COX-2 blocker. Cancer immunology, immunotherapy: CII. 2015;64(9):1175–84. 10.1007/s00262-015-1723-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979;63(2):175–9. . [PubMed] [Google Scholar]
- 50.Akagi T, Kato H, Inoue O, Sato N. A study on the optimal dose of aspirin therapy in Kawasaki disease—clinical evaluation and arachidonic acid metabolism. The Kurume medical journal. 1990;37(3):203–8. . [DOI] [PubMed] [Google Scholar]
- 51.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67(1):2–17. . [DOI] [PubMed] [Google Scholar]
- 52.Pamukcu B, Lip GY, Snezhitskiy V, Shantsila E. The CD40-CD40L system in cardiovascular disease. Annals of medicine. 2011;43(5):331–40. 10.3109/07853890.2010.546362 . [DOI] [PubMed] [Google Scholar]
- 53.Gomez I, Foudi N, Longrois D, Norel X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins, leukotrienes, and essential fatty acids. 2013;89(2–3):55–63. 10.1016/j.plefa.2013.04.004 . [DOI] [PubMed] [Google Scholar]
- 54.Daniels LB, Gordon JB, Burns JC. Kawasaki disease: late cardiovascular sequelae. Current opinion in cardiology. 2012;27(6):572–7. 10.1097/HCO.0b013e3283588f06 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.