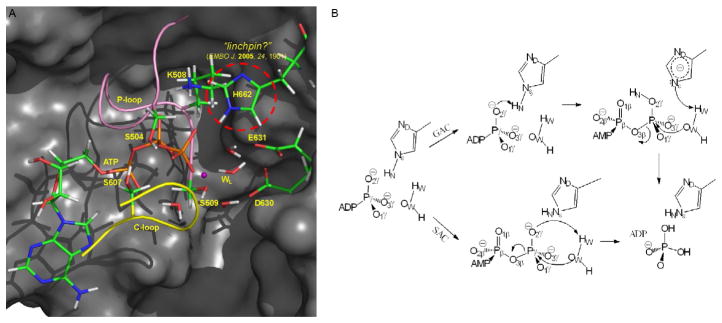
Fig. 2.
(A) Active site of HlyB-NBD. In the “mechanical linchpin” proposal, H662 holds active site residues at their catalytically competent configurations. (B) Proposed enzyme mechanisms for ATP hydrolysis in ABC transporters. In the GAC mechanism, H662 serves as a “chemical linchpin” that explicitly participates in catalysis by providing proton relay. By contrast, in the SAC mechanism, a proton is directly transferred from the catalytic water to the γ-phosphate of ATP.