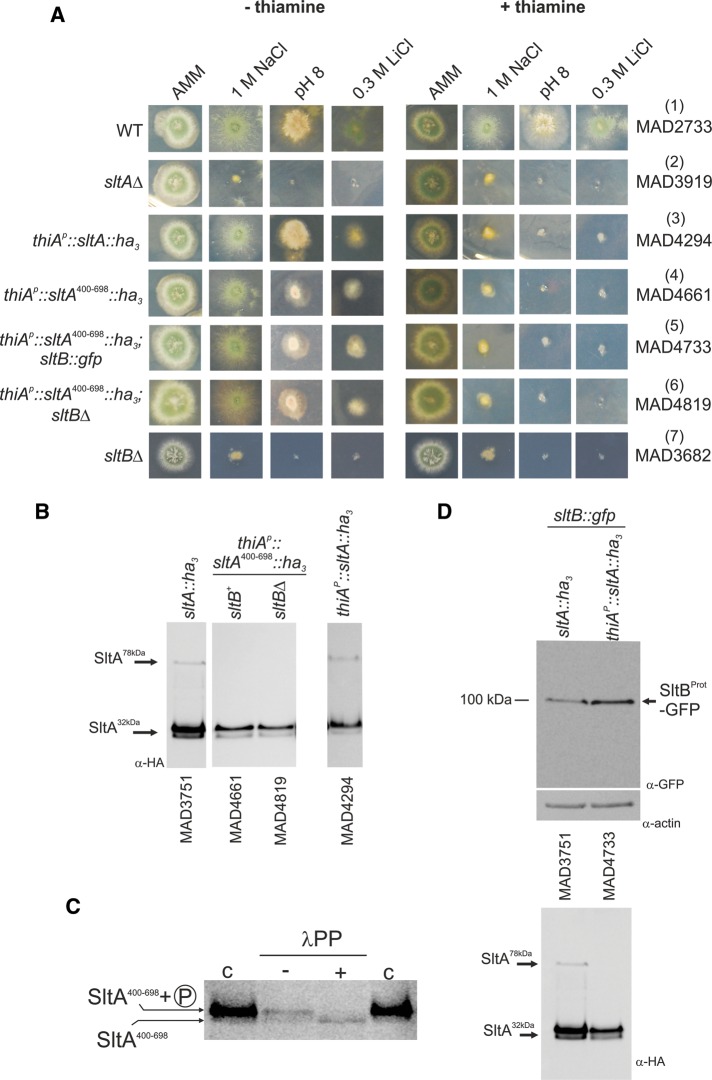
FIGURE 3:
Investigation of the role of SltA32kDa forms. (A) Conditional expression of SltA chimeras driven by the thiamine-repressible thiAp promoter. Addition of 100 μM thiamine to AMM prevents expression of thiAp. When SltA fusions were expressed through thiAp as sole source of this transcription factor, the presence of thiamine led to a null sltA phenotype for sensitivity to high concentrations of sodium or lithium and to alkalinity but a nearly WT phenotype in the absence of vitamin B1. (B) Western blots detect the three forms of SltA in strain MAD4294 (thiAp::sltA::ha3) in the absence of thiamine. Two bands having almost identical mobilities to the SltA32kDa bands are detectable when SltA400–698-HA3 is expressed. The absence of SltB has no effect on the presence or mobility of SltA400–698-HA3 bands. (C) Dephosphorylation assay of extracts from strain MAD4661 expressing the SltA400–698-HA3 fusion. Treatment with λ-protein phosphatase (λPP) results in loss of the low-mobility band and increases the intensity of the high-mobility band for the SltA400–698-HA3 fusion, demonstrating phosphorylation of this chimera (indicated on the left); c, contains total crude protein extracts obtained using the alkaline lysis procedure, resulting in a higher level of detectable SltA400–698-HA3 forms. (D) Western blots showing the presence of the 100-kDa truncated SltB-GFP form when the SltA400–698-HA3 fusion was expressed. Actin was used as loading control. The SltA-HA3 forms in those protein extracts are shown below.