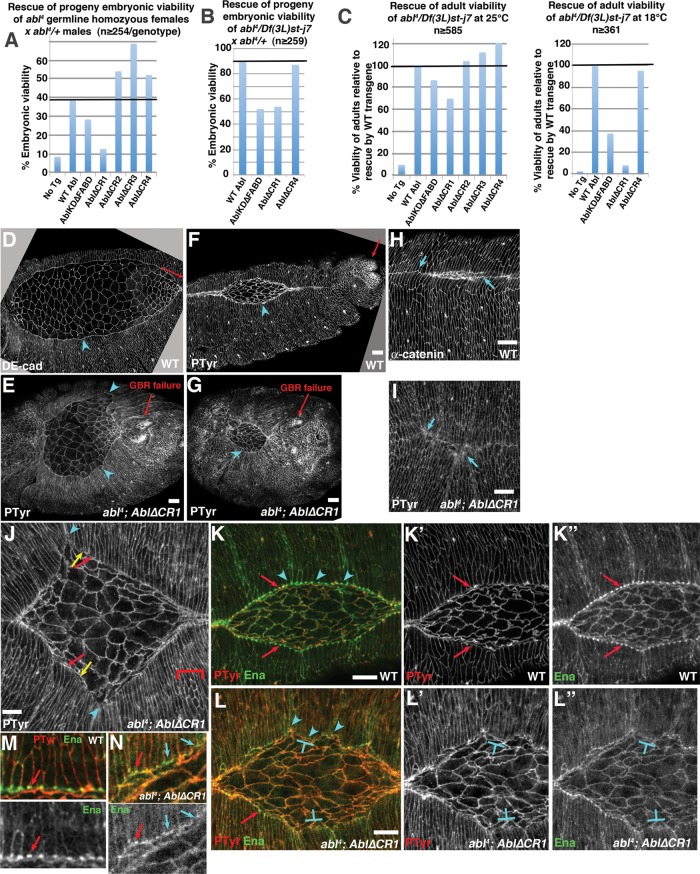
FIGURE 9:
CR1 with the PXXP motif is critical for Abl function in morphogenesis. (A, B) Rescue of embryonic viability of ablMZ mutants from abl4 germline homozygous (A) or abl4/Df(3L)st-j7 (B) mothers (50% of progeny are paternally rescued). (C) Rescue of adult viability of abl4 zygotic mutants at indicated temperatures, normalized to AblWT rescue. Full data for A–C are given in Table 1. (D–N) Stage 13–14 embryos, anterior left, dorsal up. (D–I) Embryos stained to visualize cell–cell junctions. Relative to wild type (D, F, H), Abl∆CR1 (E, G, I) fails to fully restore germband retraction (E, G, arrows) or a straight LE (D, F vs. E, G, arrowheads). (H, I) Even Abl∆CR1 mutants that do close have severe dorsal midline discontinuities (I, arrows) relative to the straight midline in wild type (H, arrows). (J) Abl∆CR1 mutants have the same large-scale defects at the LE (arrowheads), splayed open (red arrows; quantitated in Table 2) or hyperconstricted (yellow arrows) LE cells and failure of epidermal cells to elongate (brackets) seen in ablMZ mutants (Figure 6). (K–N) Abl∆CR1 fails to rescue LE Ena localization. (K, M) Close-ups. Wild type. The LE is straight (arrowheads), and Ena localizes uniformly to LE “dots” (arrows). (L, N) Close-ups. Abl∆CR1. The LE is wavy (arrowheads), and although Ena localizes to LE dots in occasional cells (red arrows), its localization is reduced and less well confined to the LE dots in most cells (T-shapes, blue arrows). Scale bars, 10 μm. In D–N, embryos are progeny of a cross in which mothers had germlines homozygous for the null allele abl4 and fathers were abl4/+. Thus all embryos were maternally mutant for endogenous abl, and half were maternally and zygotically mutant. However, in this figure, mutants scored were selected to be maternally and zygotically mutant using a GFP-marked Balancer chromosome.