Table 1.
Optimization of Reaction Parameters[a]
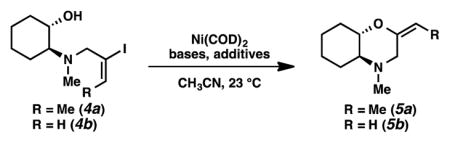
| ||||||
|---|---|---|---|---|---|---|
| entry | substrate | conc(M) | Ni mol % | base | additive | yield (%)[b] |
| 1[c] | 4a | 0.02 | 50 | Et3N (10 equiv) | – | 53 |
| 2[c] | 4b | 0.02 | 50 | Et3N (10 equiv) | – | 42 |
| 3[c] | 4a | 0.02 | 35 | Et3N (10 equiv) | – | 42[d] |
| 4[c] | 4a | 0.02 | 35 | Cs2CO3 (3.0 equiv) | – | 30[d] |
| 5[c] | 4a | 0.02 | 35 | K3PO4 (3.0 equiv) | – | 34[d] |
| 6 | 4b | 0.10 | 20 | Et3N (1.0 equiv) | DABCO (1.0 equiv) | 69 |
| 7 | 4b | 0.10 | 20 | Et3N (1.0 equiv) | CsF (1.0 equiv) | 51 |
| 8 | 4a | 0.04 | 20 | DABCO (1.0 equiv) | – | 24 |
| 9 | 4a | 0.04 | 20 | DABCO (2.0 equiv) | – | 50[d] |
| 10 | 4b | 0.15 | 5 | Et3N (1.1 equiv) | Zn (2.0 equiv) | 84 |
Reactions were performed in a N2-filled glove box.
Yield of isolated product.
DMF (0.04 M) was used as a co-solvent.
The reaction proceeded with incomplete conversion of starting material.
