Supplemental Digital Content is available in the text
Abstract
Infective endocarditis (IE) is an uncommon but potentially devastating disease. Recently published data have revealed a significant increase in the incidence of IE following the restriction on indications for antibiotic prophylaxis as recommended by the revised guidelines. This study aims to reexamine the basic assumption behind the rationale of prophylaxis that dental procedures increase the risk of IE.
Using the Longitudinal Health Insurance Database of Taiwan, we retrospectively analyzed a total of 739 patients hospitalized for IE between 1999 and 2012. A case-crossover design was conducted to compare the odds of exposure to dental procedures within 3 months preceding hospitalization with that during matched control periods when no IE developed.
In the unadjusted model, the odds ratio (OR) was 0.93 for tooth extraction (95% confidence interval [CI] 0.54–1.59), 1.64 for surgery (95% CI 0.61–4.42), 0.92 for dental scaling (95% CI 0.59–1.42), 1.69 for periodontal treatment (95% CI 0.88–3.21), and 1.29 for endodontic treatment (95% CI 0.72–2.31). The association between dental procedures and the risk of IE remained insignificant after adjustment for antibiotic use, indicating that dental procedures did not increase the risk of IE.
Therefore, this result may argue against the conventional assumption on which the recommended prophylaxis for IE is based.
INTRODUCTION
Infective endocarditis (IE) is an uncommon but potentially devastating disease, with an estimated annual incidence ranging from 2 to 7.9 per 100,000 individuals per year1,2 and a short-term mortality of 10% to 30%.3 Through the breakdown of mucocutaneous barriers and induction of bacteremia, dental therapy and other invasive procedures have been linked to seeding of heart valves and the development of IE.4–6 Since the publication of the American Heart Association (AHA) guidelines in 1955, it has been conventionally considered appropriate to prevent IE by prophylactic administration of antibiotics before procedures believed to cause bacteremia.7 However, the evidence supporting the effectiveness of antibiotic prophylaxis was poor, deriving solely from animal studies, case series, and assessments of bacteremia risk.8–10 Notably, the AHA guidelines in 1997 did acknowledge that most IE cases are not attributable to bacteremia resulting from certain invasive procedures, but rather random bacteremia from routine daily activities such as tooth brushing or chewing, and thus suggesting that prophylaxis may only prevent a small number of cases of IE.11 These guidelines also recognized the potential adverse effects and medical-legal risks associated with prophylaxis. In the absence of a robust evidence base, growing doubts with respect to this widely accepted practice12–16 led to a major revision of the AHA guidelines in 2007, narrowing the indications for antibiotic prophylaxis to a smaller population of at-risk individuals. Furthermore, the 2008 guidelines from the National Institute of Health and Clinical Excellence (NICE) recommended that antibiotic prophylaxis be abandoned in most situations.
The AHA Committee had expected such substantial changes to stimulate prospective studies on IE prophylaxis. Recently, a meticulous analysis of epidemiological data by Dayer et al reported a significantly increased incidence of IE in England (0.11 cases per 10 million people per month) that appeared to correspond with the NICE recommendations to cease antibiotic prophylaxis.17 These data again urge the use of appropriate clinical trials to reevaluate the effectiveness of IE prophylaxis. However, a large population base is required to obtain valid results for such an uncommon disease; a multicenter prospective randomized controlled trial is still lacking. As an alternative approach, investigating the link between dental procedures and the risk of IE may assist in justifying or refuting this practice. Few epidemiological studies have been carried out in this area.8,18,19 In these studies, however, IE risk associated with different types of dental procedures was estimated based on small sample sizes. Therefore, this study aims to evaluate the association between dental procedures and the acquisition of IE in a large population-based cohort using a case-crossover design.
METHODS
Data Source
The Longitudinal Health Insurance Database (LHID) 2000 is a computerized database that contains claims data of a cohort comprising 1 million subjects randomly selected from people insured by Taiwan's National Health Insurance (NHI) program. Taiwan's NHI is a single-payer, compulsory healthcare program, in which over 23 million people, equivalent to 96% of Taiwan's population, have enrolled by the end of 1996.20 The enrollment rate has reached 99.9% as of 2013. More than 90% of all health care institutions have been NHI-contracted since the inception of NHI. The LHID contains claims for hospital admissions and outpatient care and registry for beneficiaries from 1996, thereby providing longitudinal patient-level data on medical diagnosis, procedures, medical equipment use, and prescription drugs. The LHID is representative of all NHI beneficiaries and entire population of Taiwan in terms of age and sex. Personal identification information in the database was scrambled before being released in order to protect the privacy of patients and health care providers. The institutional review board of National Taiwan University Hospital has approved of this study.
Study Design
Using the LHID 2000, we did a population-based study with a case-crossover design, which is suitable for assessing the risk of acute outcomes in relation to intermittent exposures with transient effects.21,22 The case-crossover design includes only cases, that is patients who experienced the outcome events, and each case serves as his or her own control. For each patient, exposure frequency in a specified time period preceding the outcome event (case period) is compared with exposure distribution during other times when the outcome event did not occur (control periods). This self-matching design thus avoids the control selection bias and the confounding by measured and unmeasured risk factors that are time invariant within subjects but differ between subjects.
Study Subjects
Within the LHID cohort, we identified patients who had a first hospitalization with a discharge diagnosis of IE between January 1, 1999 and December 31, 2012, using codes from the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM codes 421.0, 421.1, and 421.9). The date of hospital admission served as the index date. We searched for data back to 1996 to exclude prior hospital admissions with IE. Patients were excluded if they were less than 18 years old at the time of hospitalization. To ensure a 2-year claims history, we excluded patients who did not enroll in NHI 2 years before their index date.
Case and Control Periods
Figure 1 illustrates the time frame of case and control periods in this case-crossover analysis. For each patient, we defined a period of 12 weeks preceding the index date as the case period A. This prehospitalization “at risk” period of 12 weeks had been a time interval frequently used in the literature,18,19 although the incubation period between bacteremia and the onset of symptoms of IE was estimated to be 7 to 14 days.10,18 The rationale for using this time frame was to take into account the prolonged duration of symptoms. In a previous study, the mean duration of symptoms in patients with IE was 49.6 days, and only 24.7% of patients were hospitalized within 10 days of symptom onset.23 We defined a second 12-week period starting from days 85 before the index date as the case period B, which was used for the purpose of sensitivity analysis, based on a prior assumption that the IE risk would less likely manifest in relation to dental procedures occurring more than 13 weeks before the index date. Three 12-week control periods were matched to each case period. For both case periods A and B, the control period ended 12 weeks before the start of its corresponding case period to prevent carryover effects.
FIGURE 1.
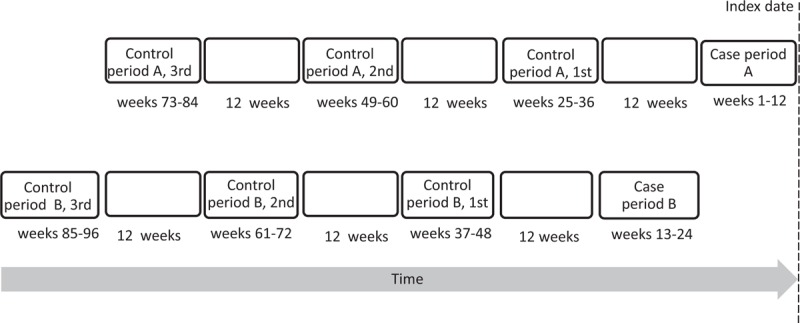
Time frame of the case and the control periods. The case-crossover design includes only cases, that is, patients who experienced the outcome events, and each case serves as his or her own control. For each patient, exposure frequency in a specified time period preceding the outcome event (case period) is compared with exposure distribution during other times (control periods).
Exposure to Dental Procedures
The dental procedures of interest are 5 common dental services provided in both inpatient and outpatient settings, including tooth extraction, surgery, dental scaling, periodontal treatment, and endodontic treatment. The procedures are listed in Supplementary Table 1, http://links.lww.com/MD/A477.
Data Analysis
To describe the characteristics of patients with IE, we presented the distribution of age, sex, and comorbidities. A patient was identified as having a comorbidity if, within 2 years before the index date, he or she had a ICD-9-CM diagnosis code of that comorbidity on at least 2 outpatient claims or any inpatient claim (Supplementary Table 2, http://links.lww.com/MD/A477). We also analyzed antibiotics related to dental procedures, defined as a prescription of antibiotics on the same date on which claims were made for dental procedures during the case periods and their corresponding control periods. Antibiotics included in the analysis were listed in Supplementary Table 3, http://links.lww.com/MD/A477.
For each of the 5 categories of dental services, we described exposure frequency during the case periods and the matched control periods. The exposures are dichotomous variables. Patients were exposed to a dental service if they had any inpatient or outpatient claim for that dental service during each specific time period.
We used the conditional logistic regression to compare the likelihood of exposure to dental procedures during case period A versus its matched control periods. The model yielded matched odds ratios (ORs) and 95% confidence intervals (CIs), which can be estimated by the ratio of the number of discordant pairs with exposed case period to the number of discordant pairs with nonexposed case periods. To adjust for potential time-varying confounders, we included antibiotics related to dental procedures in the multivariable models. We performed the adjusted and unadjusted models for each category of dental services. All these analyses were repeated using case period B and its matched control periods. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Between 1999 and 2012, 739 patients had a first recorded hospitalization with IE. Included in our analysis were 713 patients who were 18 years of age and above and have enrolled in NHI at least 2 years before the index date. The estimated incidence was 7.03 per 100,000 person years. Patient characteristics are listed in Table 1. The mean age was 58.0 ± 19.8 years; 43.8% of patients were 65 years and older. Men accounted for 65.1% of all patients. Proportions of prescriptions of dental procedure-associated antibiotics were similar between case period A and the control periods (2.8% vs 2.6%). The proportions in case period B and the matched control periods were 2.5% and 2.7%, respectively.
TABLE 1.
Characteristics of 713 Patients With Infective Endocarditis, 1999 to 2012
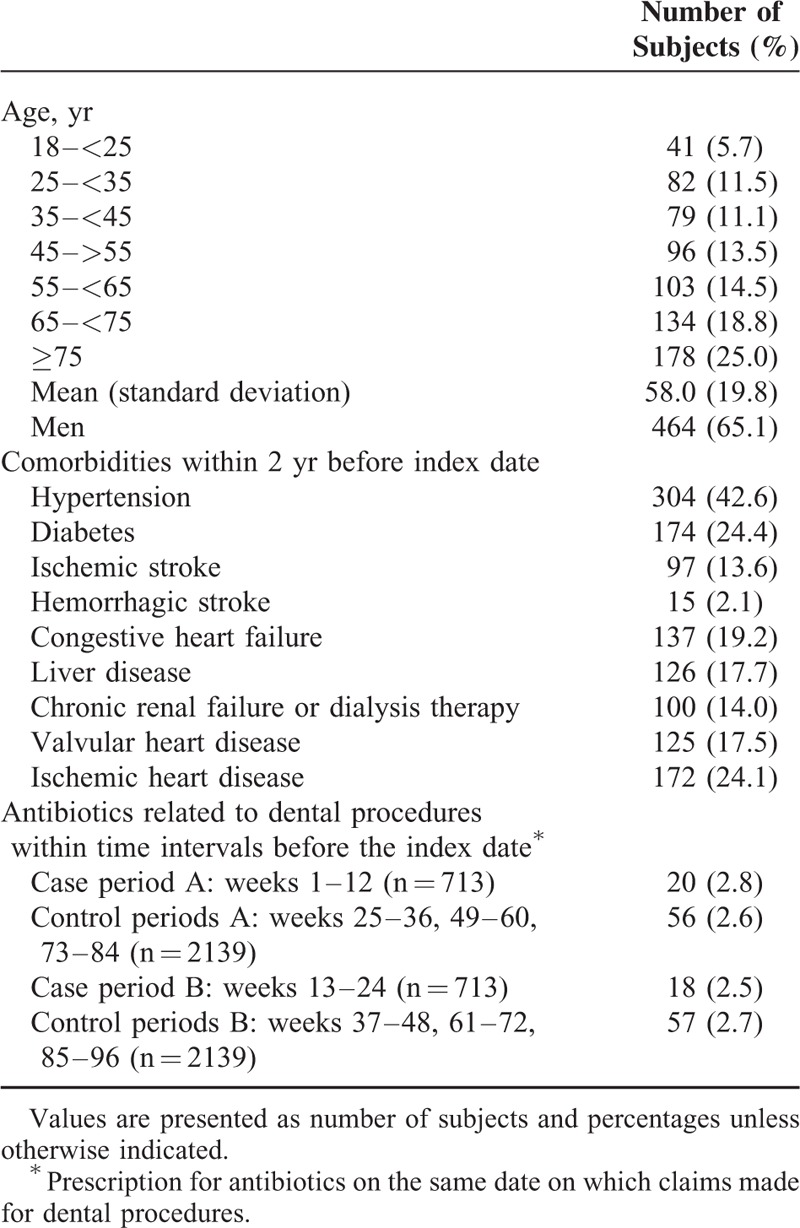
Table 2 shows the distribution of concordant and discordant matched pairs with respect to the presence or absence of exposures to dental procedures. The proportions of exposure within 12 weeks before hospitalization for IE (case period A) were 2.7% for tooth extraction, 0.8% for surgery, 3.9% for dental scaling, 2.4 % for periodontal treatment, and 2.4% for endodontic treatment. The corresponding proportions for the matched control periods were 2.6%, 0.5%, 4.3%, 1.5%, and 1.9%, respectively.
TABLE 2.
Concordant and Discordant Pairs of Exposures to Dental Procedures in Patients With Infective Endocarditis
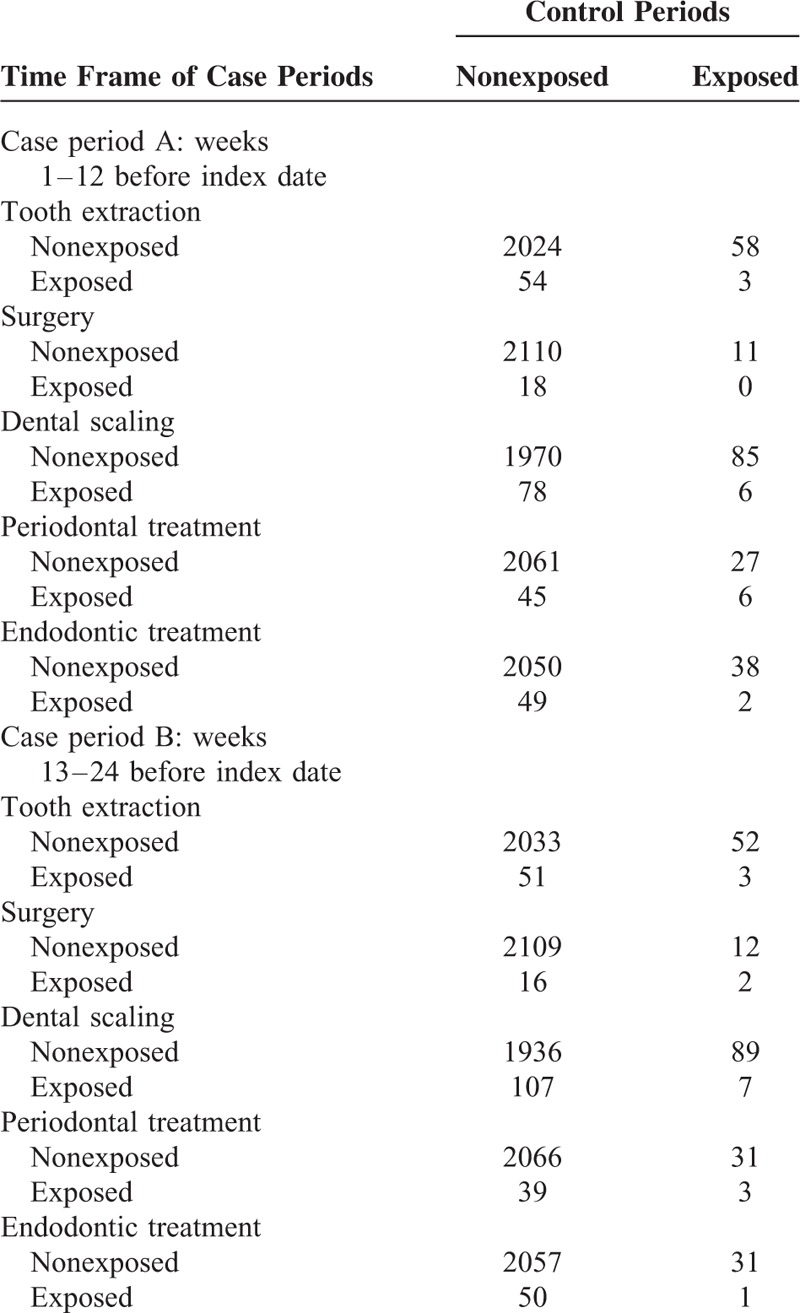
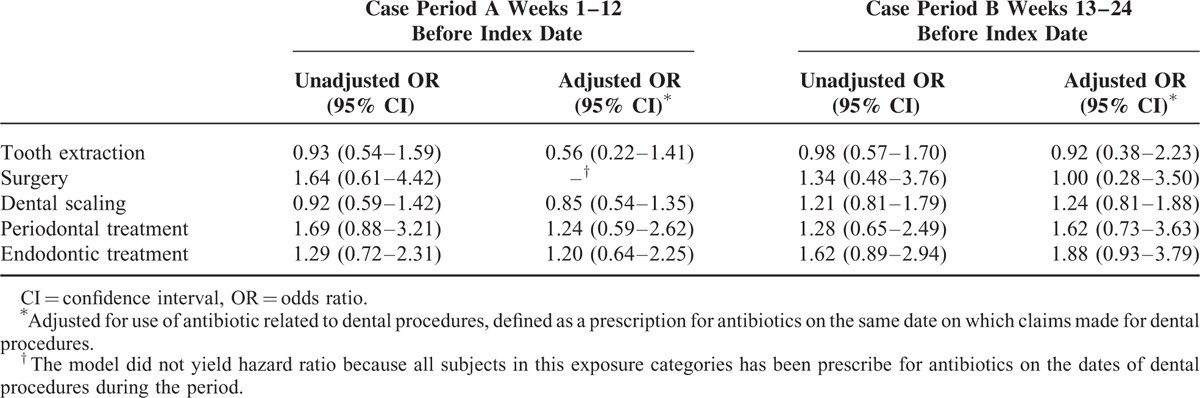
Table 3 presents the unadjusted and adjusted ORs and 95% CIs for the association between use of dental services and IE admission. The odds of exposures to all dental services were not significantly different between case period A and its matched control periods. In the unadjusted model, the ORs were 0.93 (95% CI 0.54–1.59) for tooth extraction, 1.64 for surgery (95% CI 0.61–4.42), 0.92 for dental scaling (95% CI 0.59–1.42), 1.69 for periodontal treatment (95% CI 0.88–3.21), and 1.29 for endodontic treatment (95% CI 0.72–2.31). We also did not observe appreciably increased or reduced risk associated with exposure to dental procedures when using the time window of weeks 13 to 24 before index date (case period B). The results did not change significantly after adjusting for prescription of antibiotics.
TABLE 3.
Association Between Exposures of Dental Procedures and Hospitalization With Infective Endocarditis
DISCUSSION
Using a case-crossover design, we found that the odds of exposure to dental procedures in the 3-month case period immediately preceding the admission for IE were not significantly different from those in the earlier control periods. The results remained the same even after adjusting for the use of antibiotics and further examining a second case period of 3 months preceding the first one. Our data suggest that dental procedures do not significantly contribute to the risk of IE, arguing against the rationale behind the use of antibiotic prophylaxis for dental procedures.
The underlying rationale of IE prophylaxis can be summarized as a 3-part theory: bacteremia leads to IE in at-risk patients with valvular or other cardiac abnormalities; bacteremia frequently occurs in consequence of certain invasive procedures; and, as shown in animal studies, antibiotics administered before microbial challenge can reduce the risk of IE. Despite that these factors may remain valid, collectively they neither compensate for the lack of evidence documenting the development of IE following invasive procedures in human beings in vivo, nor demonstrate a benefit from the use of prophylaxis.10 In fact, a direct causal relationship between dental procedures and IE has never been proven. Reportedly, bacterial inoculum of 1 × 108 colony-forming units per milliliter is required to consistently produce experimental endocarditis.24,25 In contrast, the intensity of bacteremia in humans is of the order of 1 × 101 or 1 × 102, which does not extrapolate easily to dental procedures as a cause of IE.26 Numerous data exist on bacteremia of varying incidence, magnitude, and duration following different types of dental procedures. Given the wide variation of reported data between studies, calculations of the absolute risk of IE resulting from a specific dental procedure would be difficult. Pallasch et al reported the estimates: mitral valve prolapse (MVP), 1 per 1.1 million procedures; presence of a prosthetic cardiac valve, 1 per 114,000; and previous IE, 1 per 95,000 dental procedures.27,28 These calculations implicate that dental or other procedures cause an extremely small fraction of cases of IE and that prophylaxis, even if 100% effective, could only prevent a small number of cases.
In a study by van der Meer et al, 23% of 275 patients with IE had undergone a procedure with an indication for prophylaxis within 180 days of onset, and in only 11.3% of the patients the procedures had been within 30 days of onset.29 Guntheroth extracted from published reports that the prevalence of dental extractions within 2 months preceding onset of IE was surprisingly low, only 3.6% for 1322 cases.30 Studies suggest that the incubation period of IE is usually 7 to 14 days for viridans group streptococci or enterococci, with 78% of cases occurring within 7 days of bacteremia and 85% within 14 days.31 It is likely that the association between dental procedures and IE has been overemphasized by some reports of cases of IE that were incorrectly attributed to these procedures for an incubation period of longer than 2 weeks. Furthermore, bacteremia from routine daily activities, such as chewing food, tooth brushing, and flossing, is remarkably more common than from dental procedures. Guntheroth estimated a cumulative exposure of 5370 minutes of bacteremia in dentulous patients resulting from chewing food and oral hygiene measures over a 1-month period, compared with a transient bacteremia of 6 to 30 minutes following a single tooth extraction considered to be the most likely bacteremia-inducing dental procedure.30 As reported by Roberts, the cumulative risk of bacteremia over 1 year from routine daily activities is 5.6 million times greater than that from a single tooth extraction.26 Given the far higher cumulative risk of bacteremia resulting from routine daily activities, it would be difficult to determine whether the bacteremia that provoked IE originated from these routine activities or from a dental procedure during the same period. In other words, the association of dental procedures and acquisition of IE might be coincidental, even performed within a short incubation period.
So far, no prospective, randomized, placebo-controlled trial has been conducted to support or reject the use of antibiotic prophylaxis. Reports of prophylaxis failure32,33 and data from some case-control studies8,19,33 have challenged the rationale for prophylaxis that dental procedures increase the risk of IE. In a population-based case-control study of 273 cases of IE, Strom et al reported that MVP, congenital and rheumatic heart disease, and previous valve surgery were risk factors of IE, but not dental treatment. They concluded that few cases of IE could be prevented with prophylaxis even with 100% effectiveness.8 In another case-control study of 171 cases of IE by Lacassin et al, dental procedures were not associated with an increased risk.19 Van der Meer reported in another case-control study that among the patients eligible for prophylaxis, 5 out of 20 cases of IE developed in spite of having received antibiotic prophylaxis, suggesting that prophylaxis might not be effective.33 However, these case-control studies are prone to control selection bias, which can result in biased estimates of odds ratio. The wide variation in the types and severity of cardiac abnormalities calls for a large population base per cohort to obtain specific matched control subjects.10 Therefore, the case-crossover design of the present study, in which each patient serves as his or her own control, may avoid the selection bias in this setting. We found that the odds of exposure to dental procedures over a 3-month period immediately preceding onset of IE were not significantly different from the earlier control periods, and the result remained the same after controlling for prescription of antibiotics. Using a similar case-crossover design in a study of 170 patients with IE, Porat Ben-Amy et al found that the number and type of dental procedures performed during the 3-month before admissions for IE were not statistically different from any earlier 3-month control periods.18 Our analysis is consistent with the prior studies in demonstrating that invasive dental procedures of different types do not seem to be a risk factor of IE, arguing against that basic assumption behind the use of antibiotic prophylaxis before those treatments.
This retrospective study was based on the LHID of Taiwan and has several inherent limitations. Firstly, the LHID contains little clinical data. The enrollment of patients with IE relied on ICD-9-CM codes but not based on the Duke criteria. Incorrect diagnosis or coding may lead to misclassification bias. The data regarding the causative microorganisms and the affected valve were not provided. Secondly, even though we used a large cohort accumulating approximately 10,146,800 person-years to identify IE patients, the statistical power might still be insufficient to detect a small or moderate association with rare exposures such as surgery. Lastly, the case number of IE was small, and only 17.5% of the study population were patients with valvular heart disease. We could not further analyze the relationship between dental procedures and IE risk among subjects with specific types of valvular disease or valve surgeries. Notably, we did not determine the efficacy of antibiotic prophylaxis. The negative result in this study argued against dental procedures as a predisposing factor of IE even after controlling for prescription of antibiotics. This could neither directly support nor refute the use of prophylaxis, although we believe that the number needed to treat should be very large and the benefit of prophylaxis, if any, may be negligible. Given the recently published data on significantly increased incidence of IE following the restriction of IE prophylaxis recommended by the revised international guidelines,17 further prospective randomized control trials are still warranted to determine the causal relationship of this observation as well as the effectiveness of antibiotic prophylaxis for IE.
In conclusion, using a case-crossover design, this nationwide population-based study found that dental procedures are not significantly associated with the risk of IE. This result may argue against the conventional assumption on which the rationale of prophylaxis for IE is based. We believe that the result with its implication is of great relevance to the practice of both physicians and dentists. Further confirmatory studies with larger scale or direct causal effects studies of IE are needed and the AHA guidelines for prevention of IE should to be reinvestigated accordingly.
Footnotes
Abbreviations: AHA = American Heart Association, CI = confidence interval, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), IE = infective endocarditis, LHID = Longitudinal Health Insurance Database, MVP = mitral valve prolapse, NHI = National Health Insurance, NICE = National Institute of Health and Clinical Excellence, OR = odds ratio.
P-CC and Y-CT contributed equally to this study.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: in France. JAMA 2002; 288:75–81. [DOI] [PubMed] [Google Scholar]
- 2.Que Y-A, Moreillon P. Infective endocarditis. Nat Rev Cardiol 2011; 8:322–336. [DOI] [PubMed] [Google Scholar]
- 3.Hasbun R, Vikram HR, Barakat LA, et al. Complicated left-sided native valve endocarditis in adults: risk classification for mortality. JAMA 2003; 289:1933–1940. [DOI] [PubMed] [Google Scholar]
- 4.Lowes J, Williams G, Tabaqchali S, et al. 10 years of infective endocarditis at St. Bartholomew's hospital: analysis of clinical features and treatment in relation to prognosis and mortality. Lancet 1980; 315:133–136. [DOI] [PubMed] [Google Scholar]
- 5.Shinebourne E, Cripps C, Hayward G, et al. Bacterial endocarditis 1956-1965: analysis of clinical features and treatment in relation to prognosis and mortality. Br Heart J 1969; 31:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandell GL, Kaye D, Levison ME, et al. Enterococcal endocarditis: an analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med 1970; 125:258–264. [DOI] [PubMed] [Google Scholar]
- 7.Jones T, Baumgartner L, Bellows M, et al. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1955; 11:317–320. [Google Scholar]
- 8.Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis: a population-based, case-control study. Ann Intern Med 1998; 129:761–769. [DOI] [PubMed] [Google Scholar]
- 9.Strom BL, Abrutyn E, Berlin JA, et al. Risk factors for infective endocarditis oral hygiene and nondental exposures. Circulation 2000; 102:2842–2848. [DOI] [PubMed] [Google Scholar]
- 10.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116:1736–1754. [DOI] [PubMed] [Google Scholar]
- 11.Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis recommendations by the American Heart Association. Circulation 1997; 96:358–366. [DOI] [PubMed] [Google Scholar]
- 12.Van der Meer JT, Thompson J, Valkenburg HA, et al. Epidemiology of bacterial endocarditis in the Netherlands: I. Patient characteristics. Arch Intern Med 1992; 152:1863–1868. [DOI] [PubMed] [Google Scholar]
- 13.Wahl MJ. Myths of dental surgery in patients: receiving anticoagulant therapy. J Am Dent Assoc 2000; 131:77–81. [DOI] [PubMed] [Google Scholar]
- 14.Durack DT. Antibiotics for prevention of endocarditis during dentistry: time to scale back? Ann Intern Med 1998; 129:829–831. [DOI] [PubMed] [Google Scholar]
- 15.Oliver R, Roberts G, Hooper L. Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst Rev 2004; CD003813. [DOI] [PubMed] [Google Scholar]
- 16.Wahl MJ, Pallasch TJ. Dentistry and endocarditis. Curr Infect Dis Rep 2005; 7:251–256. [DOI] [PubMed] [Google Scholar]
- 17.Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2014; 385:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porat Ben-Amy D, Littner M, Siegman-Igra Y. Are dental procedures an important risk factor for infective endocarditis? A case-crossover study. Eur J Clin Microbiol Infect Dis 2009; 28:269–273. [DOI] [PubMed] [Google Scholar]
- 19.Lacassin F, Hoen B, Leport C, et al. Procedures associated with infective endocarditis in adults A case control study. Eur Heart J 1995; 16:1968–1974. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CH, Huang WC, Yang JS, et al. Five-year outcomes after acute myocardial infarction in patients with and without diabetes mellitus in Taiwan, 1996-2005. Acta Cardiol Sin 2013; 29:387–394. [PMC free article] [PubMed] [Google Scholar]
- 21.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health 2000; 21:193–221. [DOI] [PubMed] [Google Scholar]
- 22.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol 2014; 43:1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issa VS, Fabri J, Jr, Pomerantzeff PM, et al. Duration of symptoms in patients with infective endocarditis. Int J Cardiol 2003; 89:63–70. [DOI] [PubMed] [Google Scholar]
- 24.Bahn SL, Goveia G, Bitterman P, et al. Experimental endocarditis induced by dental manipulation and oral streptococci. Oral Surg Oral Med Oral Pathol 1978; 45:549–559. [DOI] [PubMed] [Google Scholar]
- 25.Crémieux A-C, Saleh-Mghir A, Vallois J-M, et al. Influence of the pre-treatment duration of infection on the efficacies of various antibiotic regimens in experimental streptococcal endocarditis. J Antimicrob Chemother 1993; 32:843–852. [DOI] [PubMed] [Google Scholar]
- 26.Roberts GJ. Dentists are innocent! “Everyday” bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatr Cardiol 1999; 20:317–325. [DOI] [PubMed] [Google Scholar]
- 27.Pallasch TJ. Antibiotic prophylaxis: problems in paradise. Dent Clin North Am 2003; 47:665–679. [DOI] [PubMed] [Google Scholar]
- 28.Pallasch TJ, Wahl MJ. Focal infection: new age or ancient history? Endodontic Topics 2003; 4:32–45. [Google Scholar]
- 29.Van der Meer JT, Thompson J, Valkenburg HA, et al. Epidemiology of bacterial endocarditis in the Netherlands: II. Antecedent procedures and use of prophylaxis. Arch Intern Med 1992; 152:1869–1873. [DOI] [PubMed] [Google Scholar]
- 30.Guntheroth WG. How important are dental procedures as a cause of infective endocarditis? Am J Cardiol 1984; 54:797–801. [DOI] [PubMed] [Google Scholar]
- 31.Starkebaum M, Durack D, Beeson P. The “incubation period” of subacute bacterial endocarditis. Yale J Biol Med 1977; 50:49. [PMC free article] [PubMed] [Google Scholar]
- 32.Durack DT, Kaplan EL, Bisno AL. Apparent failures of endocarditis prophylaxis: analysis of 52 cases submitted to a national registry. JAMA 1983; 250:2318–2322. [PubMed] [Google Scholar]
- 33.Van der Meer J, Michel M, Valkenburg H, et al. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 1992; 339:135–139. [DOI] [PubMed] [Google Scholar]


