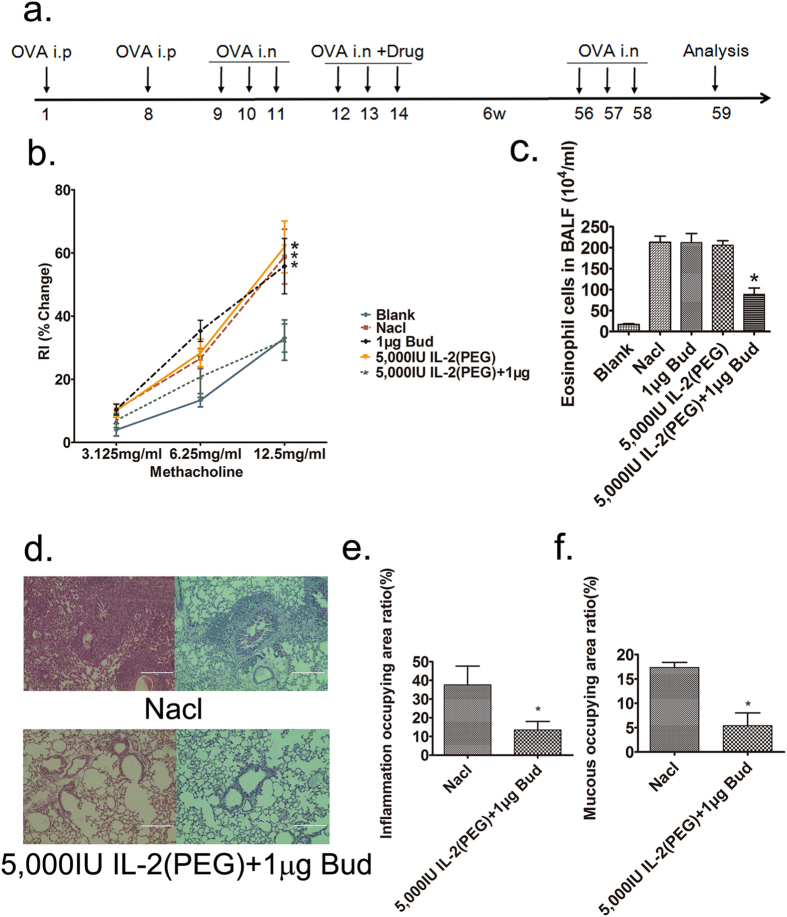
Figure 5. Manifestations of allergic airway disease 6 weeks after intratracheal use of IL-2(PEG) plus budesonide.
The therapeutic effect of intratracheal use of IL-2(PEG) plus budesonide could last for at least 6 weeks. (a) Timeline of drug intervention and analysis. Female BALB/c mice were immunized with OVA i.p on days 1 and 8, followed by intranasal (i.n) 2% OVA challenges on days 9–14. Drugs were administrated intratracheally on days 12–14. On days 56–58, mice were challenged with 2% OVA for three days again. And on day 59, mice were sacrificed and analyzed. (b–f) To prove that the amelioration of airway inflammation can last a long time, we measured AHR, eosinophil cells counts and Th2 cytokines IL-4 and IL-5 in BALF and images of lung sections (scale bars, 200 μm) in asthma model mice 6 weeks after intratracheal administration with 5,000 IU IL-2(PEG) plus 1 μg Bud for 3 days (n ≥ 4 per group). Results represent the changes in lung resistance (Rl) as a measure of AHR. *p < 0.05. (b,c,e,f) Data are presented as means ± SEM (n ≥ 4 per group and data point); here representative results from 1 of 2 experiments are shown. Treated group versus blank group (b) or Nacl group (d) by Student’s t test. (d) Left, H&E staining; right, PAS staining. i.n., intranasal; i.p., intraperitoneal. Blank group, health control mice. Nacl group, asthma model mice treated with normal saline.