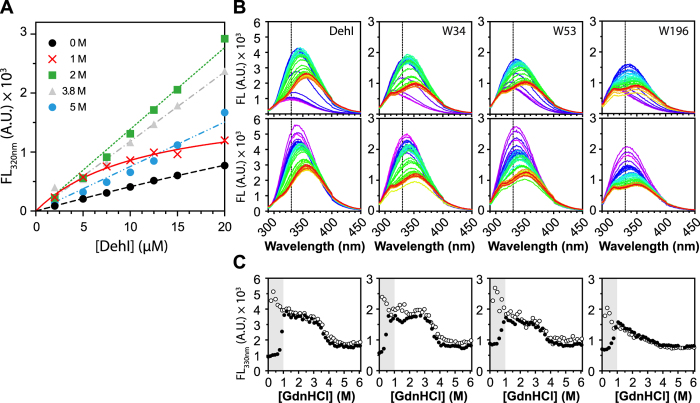
Figure 4. The dimerization formation of DehI is irreversible.
(A) Assessment of dimer dissociation by protein concentration-dependent intrinsic fluorescence. Protein concentration dependence in fluorescence change (emission wavelength 320 nm) was measured under various GdnHCl concentrations. (B) Equilibrium unfolding and refolding of DehI and variants. For unfolding analysis, 3 μM of native DehI and variants were incubated overnight at 0 to 6 M GdnHCl with a linear gradient with equal spacing (41 points). For refolding analysis, 100 μM 6 M GdnHCl-denatured DehI and variants were diluted into a final protein concentration of 3 μM within 0 to 6 M GdnHCl (41 points) and incubated overnight to refold. The emission fluorescence spectra were recorded using a fluorescence microplate reader. Each dataset was colored ramped from purple to red, representing as 0 to 6 M GdnHCl, as described in Fig. 2. An emission wavelength of 330 nm was indicated with a dashed line. (C) The fluorescence changes of the emission wavelength 330 nm for DehI and variants were plotted as a function of GdnHCl concentration, where solid circles represent for the unfolding fluorescence intensity and the open circles represent for those of refolding events. The inconsistent unfolding and refolding profiles were highlighted and revealed in a grey area with the range of 0 to 1 M GdnHCl that corresponds to the transition point of dimer dissociation.