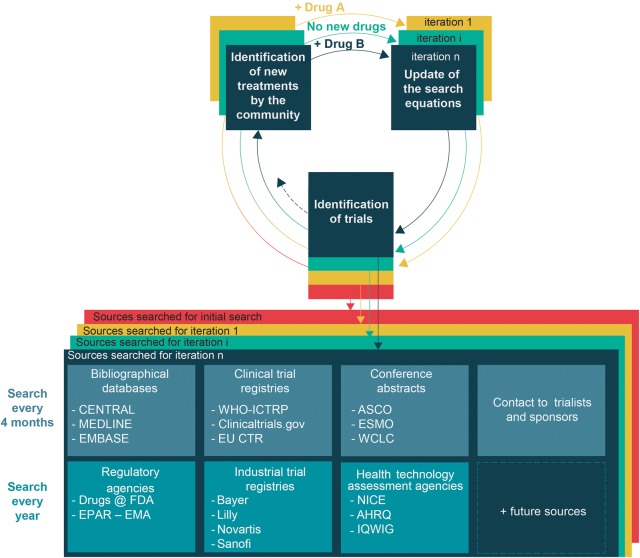
Figure 2.
Adaptive search strategy. These different sources will be searched for the initial network meta-analysis and for each iteration. A research community interested in lung cancer will identify new second-line treatments for advanced NSCLC. The search strategy (ie, specific requests for querying the different sources) will be updated over time to identify trials assessing these new treatments. We will also update this adaptive search strategy by querying new sources when they become available (eg, the OpenTrials database50). We will also consider clinical trial data sharing repositories (eg, Clinical Study Data Request or Yale University Open Data Access Project) as potential sources to identify some unpublished trials. AHRQ, Agency for Healthcare Research and Quality; ASCO, American Society of Clinical Oncology; CENTRAL, Cochrane Central Register of Controlled Trials; EU CTR, European Union Clinical Trials Register; EPAR-EMA, European Public Assessment Reports-European Medicines Agency; ESMO, European Society of Medical Oncology; FDA, Food and Drug Administration; IQWIG, Institute for Quality and Efficiency in Health Care; NICE, National Institute for Health and Care Excellence; NSCLC, non-small-cell lung cancer; WCLC, World Conference on Lung Cancer; WHO ICTRP, WHO International Clinical Trials Registry Platform.