Abstract
GATA3 is a zinc finger transcription factor that plays a crucial role in embryonic kidney development, while its precise functions in the adult kidney remain largely unexplored. Here, we demonstrate that GATA3 is specifically expressed in glomerular mesangial cells and plays a critical role in the maintenance of renal glomerular function. Newly generated Gata3 hypomorphic mutant mice exhibited neonatal lethality associated with severe renal hypoplasia. Normal kidney size was restored by breeding the hypomorphic mutant with a rescuing transgenic mouse line bearing a 662-kb Gata3 yeast artificial chromosome (YAC), and these animals (termed G3YR mice) survived to adulthood. However, most of the G3YR mice showed degenerative changes in glomerular mesangial cells, which deteriorated progressively during postnatal development. Consequently, the G3YR adult mice suffered severe renal failure. We found that the 662-kb Gata3 YAC transgene recapitulated Gata3 expression in the renal tubules but failed to direct sufficient GATA3 activity to mesangial cells. Renal glomeruli of the G3YR mice had significantly reduced amounts of platelet-derived growth factor receptor (PDGFR), which is known to participate in the development and maintenance of glomerular mesangial cells. These results demonstrate a critical role for GATA3 in the maintenance of mesangial cells and its absolute requirement for prevention of glomerular disease.
INTRODUCTION
Kidney development is regulated by the intimate interplay of a large number of transcription factors that are expressed in the urogenital primordia. Hereditary mutation of genes encoding these factors often leads to congenital renal anomalies or adult-onset kidney diseases (reviewed in reference 1). Among these pathologically important factors is Gata3, which encodes a transcription factor containing two GATA-type zinc fingers that serve as its DNA binding domain. This domain recognizes and binds to the cognate consensus motif (A/T)GATA(A/G) and is highly conserved among the six members (Gata1 to Gata6) that constitute this multigene family (2–5).
In developing embryos, Gata3 is tissue specifically expressed in several immature tissues, including the urogenital primordium (Wolffian duct and ureteric bud) as well as in the pharyngeal arches (6–9). The former gives rise to reproductive tissues and the kidney, while the latter contribute to adult craniofacial structures, including the upper and lower jaws. GATA3 is indispensable for the development of both tissues, as Gata3-null deficiency leads to kidney agenesis and lower jaw defects (10–12). Prominent GATA3 expression is also detected in the developing sympathetic nervous system, in which GATA3 participates in embryonic catecholamine biosynthesis and subsequent cardiovascular development (11, 13). Indeed, Gata3 null mutant mice die of cardiovascular defects around embryonic day 11 (e11) due to catecholamine deficiency, while this fatal defect can be rescued to late embryonic stages by pharmacological administration of catecholamine intermediates (11). Such postnatal lethality completely obscures the physiological functions of GATA3 in adult-stage organogenesis.
Human GATA3 haploinsufficiency causes a unique, dominantly inherited condition known as HDR syndrome (i.e., hypoparathyroidism, sensorineural deafness, and renal dysplasia) (14). Kidney abnormalities that have been reported to be associated with HDR syndrome include nephrotic syndrome, chronic kidney disease, and various congenital kidney anomalies, i.e., cystic kidney, renal dysplasia, hypoplasia, and agenesis (15). More recently, a clinical study reported an HDR syndrome case that is associated with glomerulonephritis (16). These results suggested that GATA3 plays a crucial role in human renal development and for maintenance of glomerular homeostasis. Despite accumulating clinical evidence, the lack of a faithful kidney disease model resulting from GATA3 deficiency has impeded postembryonic analysis.
To at least partially resolve this issue, in the present study we have generated a hypomorphic Gata3 mutant allele in which GATA3 expression is diminished to less than the haploid expression level. Utilizing various mouse mutants and transgenic animals, we demonstrate here that the level of GATA3 activity in the developing kidney must be stringently maintained for the development of glomerular mesangial cells and for prevention of glomerular disease.
MATERIALS AND METHODS
Generation of the Gata3g hypomorphic allele.
The targeted conditional knock-in GATA3-green fluorescence protein (GFP) allele was generated by inserting a cDNA encoding a GATA3-GFP fusion protein in frame at the Gata3 gene start codon in 129Sv-derived embryonic stem (ES) cells (Gata3g) (Fig. 1A). To construct the GATA3-GFP conditional targeting vector, 3.5 kb of sequence corresponding to the promoter and 5′ untranslated region (UTR) of the Gata3 gene (5′ homology region) was ligated to the GATA3-GFP cDNA, both ends of which were flanked by loxP sequences. The 3′ end of the GATA3-GFP cassette was then ligated to a neomycin resistance gene cassette that was flanked by FRT (flip recombinase target) sequences. An additional 3-kb Gata3 genomic fragment including exon 3 (for the 3′ homology region) was added at the 3′ end of the construct. Discosoma red fluorescent protein (Dsred2) DNA was inserted in exon 3 of the 3′ homology region, which was designed to generate red fluorescence upon deletion of the GATA3-GFP cDNA. The targeting vector was linearized and electroporated into R1 mouse ES cells (17); appropriately targeted ES clones (as determined by PCR) were then used to generate chimeric animals by injection of the cells into blastocysts. Chimeric animals were backcrossed to C57BL/6J mice to generate germ line Gata3g/+ progeny. The Neor cassette was then excised by crossing heterozygous mutants with mice that ubiquitously express FLP recombinase (18). All mice were handled according to the regulations of the standards for use of laboratory animals at both Tohoku University and the University of Michigan. All the animal procedures follow guidelines established for the proper conduct of animal experiments from the ministry of education, culture, sports, science and technology (MEXT) of Japan.
FIG 1.
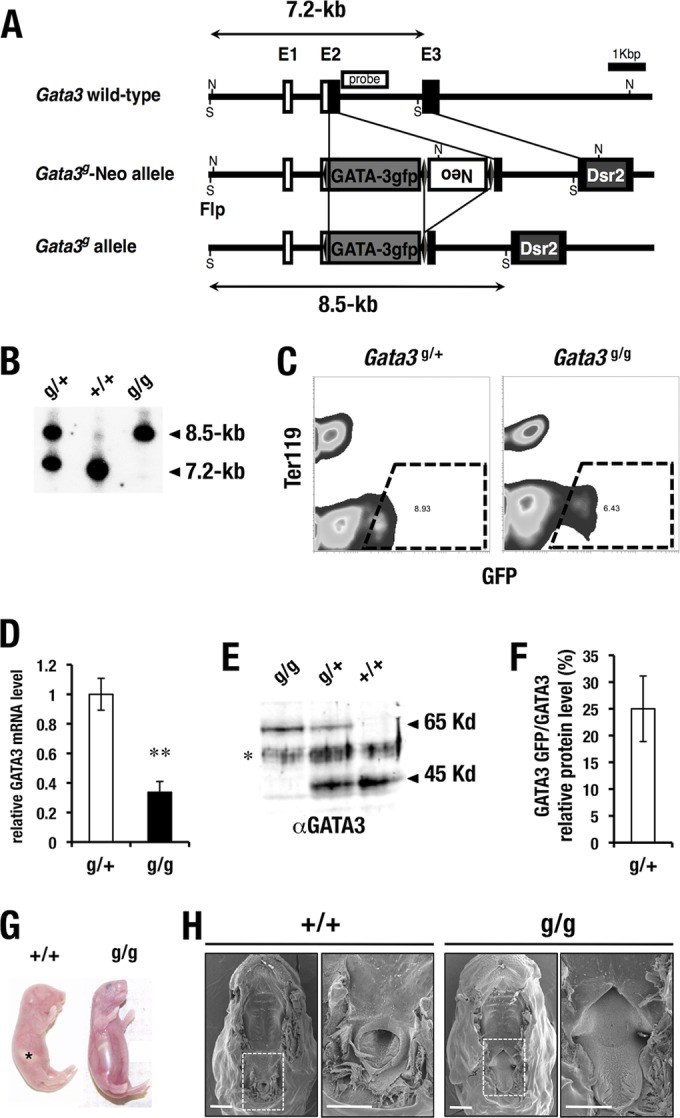
Generation of Gata3 hypomorphic (Gata3g) mice. (A) Strategy for GATA3-GFP cDNA knock-in to the Gata3 locus by homologous recombination. S and N, restriction enzyme cleavage sites for SacI and NcoI, respectively; Neo, neomycin resistance gene; Dsr2, DsRed2 (Discosoma red fluorescent protein). (B) The SacI restriction fragments detected with the 2nd intron probe in the wild type (7.2 kb) and Gata3g allele (8.5 kb) are indicated. (C) Flow cytometry analysis of kidney cells. GFP-positive GATA3-expressing cells (dotted line) from Gata3g/+ and Gata3g/g kidney were sorted and subjected to RT-qPCR analysis. (D) GATA3-GFP mRNA abundance in the Gata3g/g kidney was diminished to 35% compared with the total abundance of GATA3 and GATA3-GFP mRNA in the Gata3g/+ kidney (g/+, n = 3; g/g, n = 3); statistical significance of differences is indicated (**, P < 0.05 by Student's unpaired t test). (E) Western blot analysis using nuclear extract of kidney. Note that the 65-kDa GATA3-GFP fusion protein is detected in the Gata3g/+ and Gata3g/g samples. *, nonspecific protein. (F) GATA3-GFP-to-GATA3 protein expression ratio in the Gata3g/+ heterozygous mice. GATA3-GFP protein abundance is lower than that of normal GATA3 protein in the Gata3g/+ heterozygotes. (G) Gata3g/g mice show air-swallowing abnormality with drinking inability. Control wild-type mouse shows normal milk accumulation in the stomach (asterisk). (H) Surface electron microscope analysis shows soft palate defects in the neonate Gata3g/g mouse. Wild-type littermates show a normal palatal structure. High-magnification images are derived from the dashed inset in the low-magnification images. Scale bars, 1 mm.
Transgenic and germ line mutant mice.
The Gata3 lacZ knock-in (Gata3Z) null mutant allele has been described previously (19). The Gata3 B125lacZ yeast artificial chromosome (YAC) transgenic line (TgB125Z), which contains 662 kb of genetic information (from kb −451 to +211 of the Gata3 locus), was marked by insertion of a lacZ expression cassette at the Gata3 start codon (8). The Gata3 B125 (wild-type) YAC transgenic lines (TgB125-G3) have been described previously (9, 12). The copy number and integrity of the transgenes were determined by genomic quantitative PCR as previously described (20). Primers used for genomic quantitative PCR are listed in Table 1.
TABLE 1.
Sequences of primers used in RT-qPCR and genomic qPCR
| Gene | Sense primer | Antisense primer | Assay |
|---|---|---|---|
| Kim1 | AAACCAGAGATTCCCACACG | GTCGTGGGTCTTCCTGTAGC | RT-qPCR |
| Ngal | CAAGCAATACTTCAAAATTACCCTGTA | GCAAAGCGGGTGAAACGTT | RT-qPCR |
| Nephrin | GACCGGGACACAAGAAGCTC | GATGTCCCCTCAGCTCGAAG | RT-qPCR |
| Podocin | CATGTGTCCAAAGCCATCCA | CTTTTCCCCTTCTGCAGCAA | RT-qPCR |
| Pdgfb | ATCCGCTCCTTTGATGATCT | GAGCTTTCCAACTCGACTCC | RT-qPCR |
| Pdgfra | TCCTTCTACCACCTCAGCGAG | CCGGATGGTCACTCTTTAGGAAG | RT-qPCR |
| Pdgfrb | CGGCCTGTGACTAGAAGTCC | GAGCTTGAGGCGTCTTGG | RT-qPCR |
| Megsin | GGCGGTTCAATTTGTCTACCA | ATGTACATGCTTATGCCACCA | RT-qPCR |
| Avpr2 | GGTCTCGGTCATCCAGTAGC | CTGGTGTCTACCACGTCTGC | RT-qPCR |
| Aqp2 | CAGCTCGAAGGAAGGAGACA | GCATTGGCACCCTGGTTCA | RT-qPCR |
| Gata3 | GGTGGACGTACTTTTTAACATCGA | CCCTGACGGAGTTTCCGTAG | RT-qPCR |
| Itih 5′-UTR | CTGGACAGGCTGGAATTGAT | CAGCGGGAGCTAACAAGAAC | Genomic qPCR |
| Itih 3′-UTR | AGATAGGGCATGTGGAGTGG | AGCTCGTGCTCATTGGAGAT | Genomic qPCR |
| Gata3 KE | GGCCTACTCTTTCTGGCAAA | AGCTGATGACCCAGTGAACC | Genomic qPCR |
| Int S1-E2 for YAC | CGAAGACCTAGTGCCCTCCAGAATTTCAGG | CTTGGCGCTCAGAGACGGTTGC | Genomic qPCR |
| Gata3 3′ 100 kb | AATGCTCCTCAAATGCATCC | TGTGTGTGTTTGCCAGGAAT | Genomic qPCR |
Histological analysis and SEM studies.
Tissues were fixed overnight in 4% paraformaldehyde at 4°C and then processed for hematoxylin and eosin staining in paraffin sections. Whole-mount X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining was performed as previously described (9). For the scanning electron microscope (SEM) studies, the P0 palatal samples were processed by standard protocols (21). The images were viewed on an AMRAY 1000-B scanning electron microscope in the Microscopy and Image Analysis Laboratory, University of Michigan.
Immunohistochemistry.
Immunohistochemical analysis was performed as previously described (22). The antibodies used were αCD73 (abcam ab91086) and αSynaptopodin (abcam ab50859).
Flow cytometry.
Flow cytometry was performed as described previously (23). The fluorescence-activated cell sorter (FACS) Aria (BD Bioscience) was used to separate the GFP-positive cell population recovered from the kidneys of Gata3g/g and Gata3g/+ mice. Total RNA from GFP-positive cells was prepared with the RNeasy Micro kit (Qiagen) and subjected to quantitative reverse transcription-PCR (RT-qPCR) analysis, as described previously (20). Primers used for the RT-qPCR analysis are listed in Table 1.
Glomerular isolation and real-time RT-PCR.
Glomeruli were isolated from the mice by injection of magnetic beads that become trapped within glomerular capillaries (24). Briefly, mice were anesthetized with 1 mg/10 g body weight ketamine and 0.15 mg/10 g body weight of xylazine. Blood was washed from the animals by perfusion of the heart with Hanks' balanced salt solution (HBSS) followed by intracardiac injection of 2 × 106 Dynabeads M-450/ml in HBSS (Invitrogen). Kidneys were removed and minced on ice, followed by digestion at 37°C with 1 mg/ml collagenase and 100 U/ml DNase I for 30 min. Digested kidneys were treated twice by separation through 100-μm Falcon cell strainers, and the collected tissue was pelleted by gentle centrifugation (200 × g, 5 min). Glomeruli were isolated by three rounds of DynaMag-2 (Invitrogen) magnetic particle concentrator treatment, resuspending in HBSS after each collection. These purified glomerular preparations were briefly spun, frozen immediately on dry ice, and stored at −80°C. Total RNA was isolated using the RNeasy Micro kit (Qiagen). The RNA sample was subjected to RT-qPCR analysis, as described previously (20). Primers used for the RT-qPCR analysis are listed in Table 1.
RESULTS
Perinatal lethality in Gata3 hypomorphic mutant mice.
We wished to create a new Gata3 germ line mutant allele in which a cDNA encoding GATA3 was fused to the N terminus of GFP, with the expectation that this would allow us to trace endogenous GATA3 expression by GFP while maintaining GATA3 transcriptional activity (Fig. 1A). The GATA3-GFP protein was able to trans activate a GATA-responsive luciferase reporter gene after transient transfection into multiple cell lines. However, the transcriptional activity of GATA3-GFP protein was slightly lower than that of wild-type GATA3 depending on the transfected cell line (data not shown).
This same construct was targeted in embryonic stem cells to the first coding exon of Gata3, and germ line mutant mice expressing the GATA3-GFP fusion protein were recovered (Gata3g) (Fig. 1A and B). Importantly, no genetic information was deleted from the endogenous locus after targeted insertion of the GATA3-GFP cDNA. To address GATA3 mRNA abundance, we purified GFP-positive cells from Gata3g/+ and Gata3g/g kidneys by flow cytometry (Fig. 1C). GATA3 mRNA levels in the Gata3g/g kidneys (expressing only the fusion protein) were reduced to only 35% compared to its abundance in Gata3g/+ kidneys (expressing both GATA3 and GATA3-GFP mRNA) (Fig. 1D). Western blot analysis revealed that GATA3-GFP protein abundance was only 10% to 25% (depending on the tissue source) of the amount produced in mice with the wild-type Gata3 allele (Fig. 1E and F) (25). Therefore, homozygous mutant mice generate significantly less GATA3-GFP fusion protein than the amount of wild-type protein that normally accumulates in Gata3+/+ mice.
The homozygous Gata3g/g mice looked healthy immediately after birth, but shortly thereafter they developed severe gastrointestinal dilation due to air swallowing (Fig. 1G). We carefully examined the upper respiratory tract and eventually found that Gata3g/g mice often exhibited partial clefts in the soft palate (Fig. 1H), which presumably skewed respiratory airflow and led to the aberrant air-swallowing. Consequently, none of the homozygous mutant pups survived for more than 2 days after birth. Since Gata3-null mutants die at embryonic day 11 of secondary cardiac defects (11), these results confirm that the Gata3g fusion allele is hypomorphic, thus conferring survival of Gata3g/g pups past birth until they succumb to direct or indirect lethal effects of palatal deficiencies.
Gata3g/g mice exhibit renal abnormalities.
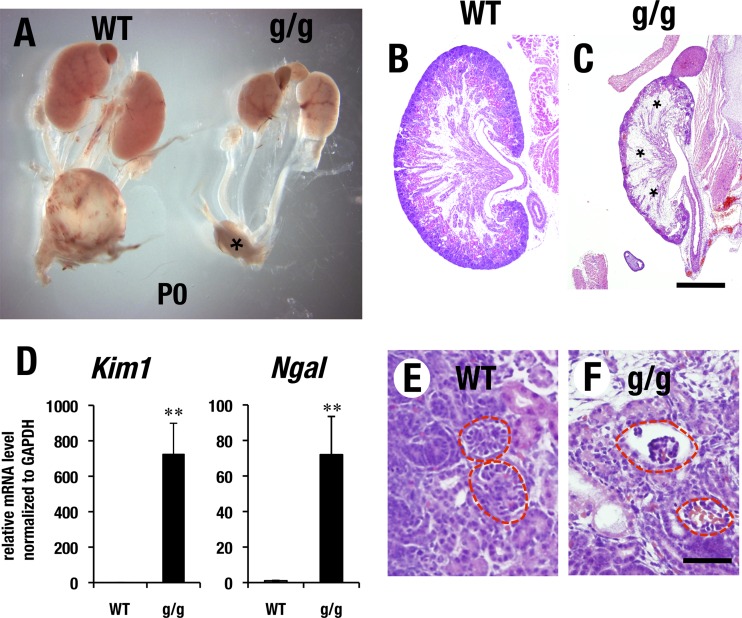
We noticed on dissection that homozygous mutant neonates had significantly hypotrophic kidneys (Fig. 2A), which was associated with a reduced number of nephrons and prominent fibrosis in comparison to wild-type control kidneys (Fig. 2B and C, asterisks). Kim1 (kidney injury molecule 1) and Ngal (neutrophil gelatinase-associated lipocalin) are widely used biomarkers for renal tubular cell damage (26). The Gata3g/g kidney exhibited robust induction of Kim1 and Ngal in comparison to the levels in wild-type kidneys (Fig. 2D). The remaining glomeruli of Gata3g/g mice had a hypoplastic morphology with diminished cellularity (Fig. 2F), whereas the control kidneys had normal glomerular development (Fig. 2E). We surmised that the hypomorphic GATA3 protein abundance (or activity) in the Gata3g/g mice was insufficient to fulfill a normal kidney developmental program and thereby produced severe renal hypoplasia and subsequent fibrosis during embryonic to postnatal development. The hypotrophic kidneys in the Gata3g/g neonates rarely generate urine in the bladder (Fig. 2A, asterisk), suggesting that a presumptive renal failure in the Gata3g/g newborns may also contribute to neonatal lethality.
FIG 2.
Gata3g/g mice exhibit renal deformity. (A) Gata3g/g neonate (P0) mouse shows atrophic kidney in comparison with wild-type control. Note that Gata3g/g mouse shows no urinary collection in bladder (asterisk). (B, C) Gata3g/g mouse shows significant thinning of renal cortex and robust renal fibrosis in the medullary region (asterisks in panel C), while the wild-type kidney shows a normal histological appearance. Scale bar, 0.5 mm. (D) Gata3g/g neonate shows robust increase of mRNA expression of renal injury markers, Kim1 and Ngal (WT, n = 5; g/g, n = 6; statistical significance of differences is indicated: **, P < 0.05 by Student's unpaired t test). (E, F) Gata3g/g kidney shows a reduced number of glomeruli in comparison with the wild-type control (circles). Remaining glomeruli in Gata3g/g kidney show diminished cellularity and often associate with enlarged glomerular capillary. Bar, 50 μm.
Gata3 flanking sequences direct lacZ reporter expression to the renal primordium and pharyngeal arch.
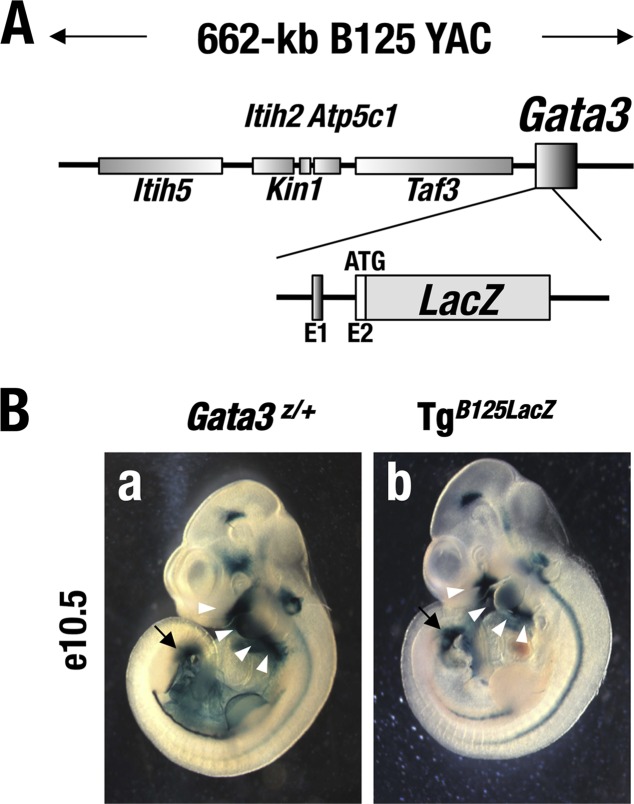
Previously, we generated transgenic mouse lines carrying a 662-kb Gata3 YAC (yeast artificial chromosome) that contains approximately 451 kb of 5′ flanking sequence and 211 kb of 3′ flanking sequence as well as the entire structural (transcribed) gene (the parental B125 YAC), but modified to include a lacZ reporter gene inserted in frame at the Gata3 translational initiation codon (here referred to as TgB125Z) (Fig. 3A) (8, 9). Most of the embryonic tissues where the lacZ reporter gene was expressed in the TgB125Z mice reflected tissues that were labeled in Gata3Z/+ germ line lacZ knock-in mutant mouse embryos at e10.5 (Fig. 3B) (9, 19). Importantly, TgB125Z mice recapitulated the Gata3 expression pattern in the urogenital primordia, i.e., in the mesonephric duct and ureteric bud region (Fig. 3B, arrows) (9). Furthermore, the B125Z YAC directs lacZ reporter gene expression to the pharyngeal arch in e10.5 embryos (arrowheads in Fig. 3B), which gives rise to craniofacial tissues at later developmental stages. Given these lacZ expression patterns, we surmised that GATA3 expression directed by the B125 YAC transgene would complement GATA3 activity in the renal primordia and the pharyngeal arch tissues and might thereby restore normal development of the kidney and maxillary palate in the Gata3g/g hypomorphic mutant mice.
FIG 3.
(A) The 662-kb B125 YAC DNA contains approximately 451 kb of 5′ and 211 kb of 3′ flanking sequences and all the exons of Gata3 gene. The lacZ cassette is inserted in the 2nd exon (9). (B) Embryonic day 10.5 Gata3Z/+ (a) and TgB125Z (b) embryos show similar lacZ expression patterns in the ureteric bud region (arrows) and the pharyngeal arch (arrowheads).
The Gata3 YAC transgene rescues neonatal lethality in the Gata3g/g hypomorphic mutant mice.
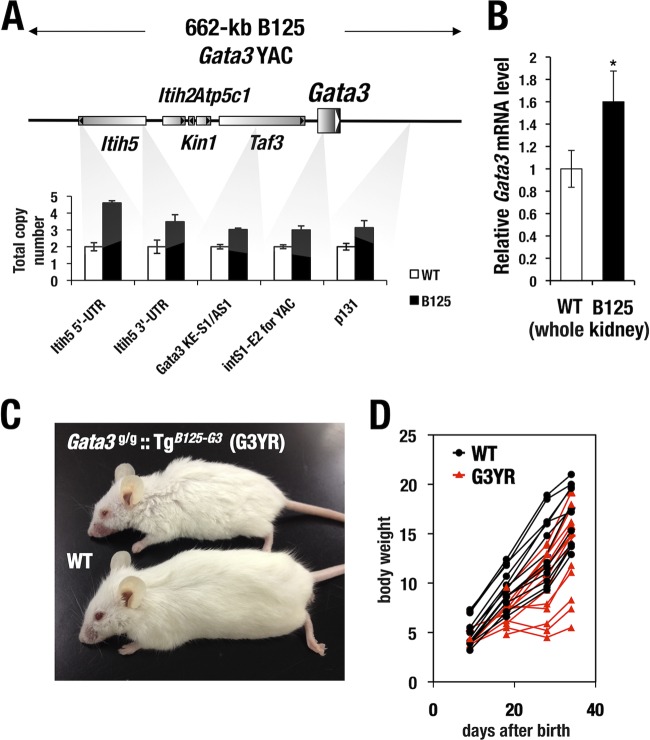
To test whether the B125 YAC could reconstitute a normal developmental program in the kidney and craniofacial tissues of the Gata3g/g mice, we generated a transgenic mouse line harboring one intact copy of the B125 YAC (TgB125-G3). After backcrossing the founder for more than 10 generations onto an ICR/CD-1 genetic background, the integrity of the B125 YAC was confirmed by quantitative PCR using 5 different primer pairs recognizing sequences within the Gata3 flanking region (Fig. 4A). We found that single-copy DNA from 300 kb 5′ to 130 kb 3′ to the Gata3 structural gene was stably transmitted (Fig. 4A). TgB125-G3 mice showed a higher level of Gata3 mRNA in the kidney than did wild-type littermate mice (Fig. 4B), indicating that the urogenital primordium-directed regulatory activity in the B125 YAC functions in the adult definitive kidney (8, 12).
FIG 4.
Analysis of the structural integrity of Gata3 YAC transgene by genomic qPCR. (A) Diagram of area around the Gata3 locus. PCR primer pairs for genomic quantitative PCR were designed at the five sites distributed from the 300-kb 5′ flanking sequence to the 130-kb 3′ flanking sequence in the Gata3 locus. The TgB125-G3 mouse line harbors a single copy of transgene for most of the part, while an additional 5′ end fragment may remain. (B) Whole-kidney sample of adult TgB125-G3 mice showed increased Gata3 mRNA expression in comparison with wild-type control (WT, n = 4; TgB125-G3, n = 5). The mRNA level is normalized with the GAPDH level. The statistical significance of the difference between WT and TgB125-G3 is indicated (*, P < 0.05; Student's unpaired t test). (C) Gross appearance of Gata3g/g::TgB125-G3 (G3YR) mouse and wild-type littermate. (D) Postnatal body weight gain of G3YR (n = 9) and wild-type (WT, n = 7) mice. Note that a certain fraction of G3YR mice show severe growth retardation.
We next crossed the TgB125-G3 mice into the Gata3g/g background to ask whether the B125 YAC could rescue the observed neonatal lethality in the Gata3g/g mice. When multiple litters of interbred mice were examined at 3 weeks after birth, 18 TgB125-G3 transgene-rescued homozygous Gata3 hypomorphic mutants (Gata3g/g::TgB125-G3) were recovered among 154 total progeny (Table 2). Thus, the YAC rescue was approximately 82% effective according to the anticipated Mendelian frequency from this complex mating. The Gata3g/g::TgB125-G3 mice showed no palatal deformity (data not shown), underscoring at least one point of successful rescue with the TgB125-G3 YAC transgene. The compound mutant Gata3g/g::TgB125-G3 mice exhibited slight growth retardation in comparison to their wild-type littermates (Fig. 4C and D). Collectively, in the YAC transgene-rescued Gata3 hypomorphic mutants, the lethality normally encountered as a consequence of homozygous Gata3 hypomorphic loss of function was successfully rescued to adulthood by the activity conferred by the TgB125-G3 transgene. We refer to these Gata3g/g::TgB125-G3 rescued mice here as G3YR mice (i.e., Gata3 YAC rescued mice) and subjected them to detailed analysis for renal function and histology.
TABLE 2.
Rescue of the Gata3g/g neonatal lethal phenotype by 662-kb TgB125-G3 YACa
| Gata3 genotype | No. of pups |
|
|---|---|---|
| Tg− | Tg+ | |
| +/+ | 24 | 21 |
| g/+ | 47 | 44 |
| g/g | 0 | 18 |
The neonate 3-week-old mice are derived from intercross between Gata3g/+ and Gata3g/+::TgB125-G3 mice.
G3YR mice exhibit pathological changes in renal glomeruli.
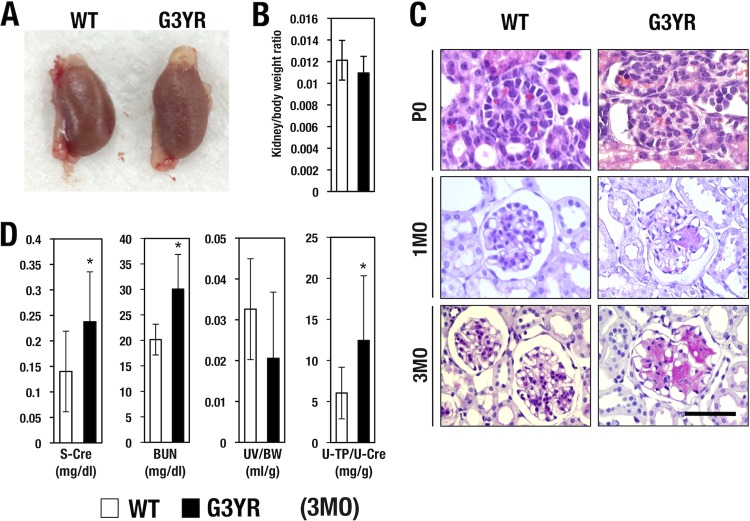
The gross size of the kidneys in the G3YR mice was comparable to that of wild-type control mice (Fig. 5A). The ratio of kidney weight to body weight of the G3YR animals was almost the same as that of their wild-type littermates (Fig. 5B). When we examined renal histology by hematoxylin and eosin (H&E) staining, we found that the glomerular and tubular structure of the newborn G3YR pups was almost normal in appearance compared to that of wild-type littermates (Fig. 5C, top panels). Of note, from 1 month after birth onward, all the G3YR mice exhibited enlarged capillaries and very dense deposition of extracellular matrix in the glomerular mesangial region (Fig. 5C, middle panels). This capillary enlargement and accumulation of mesangial matrix were progressively exacerbated by 3 months after birth (Fig. 5C, bottom panels). These pathological features are compatible with mesangial proliferative glomerulonephritis in the renal glomerulus (27). While this type of glomerular nephritis is often observed in diabetic nephropathy, the steady-state serum blood sugar levels of the G3YR mice were comparable to that of wild-type mice (data not shown).
FIG 5.
(A) Kidneys of G3YR (Gata3g/g::TgB125-G3) mouse and wild-type (WT) littermate show comparable sizes. (B) G3YR (n = 9) and WT (n = 7) mice show comparable kidney weights normalized to body weight. (C) H&E staining of renal glomerulus. At the postnatal day 0 (P0) stage, the two genotypes of mice show similar histological appearances. At 1 month (1MO) afterward, the G3YR mice show reduced glomerular cellularity associated with dilated capillary and robust accumulation of mesangial matrix. Wild-type control mice show a normal histological appearance. Scale bar, 50 μm. (D) G3YR mice (n = 9) show increased levels of serum creatinine and blood urea nitrogen in comparison with the wild-type littermates (n = 7). Urine volumes normalized to body weight for the two genotypes are comparable. Statistical significances in differences are indicated (*, P < 0.05; Student's unpaired t test).
In agreement with our initial diagnosis of progressive mesangial proliferative glomerulonephritis, the G3YR mice exhibited increased serum creatinine (s-Cre) and blood urea nitrogen (BUN) (Fig. 5D). While urine volume was not significantly altered, urine protein levels, when normalized to urine creatinine levels, were significantly elevated in the G3YR mice (Fig. 5D). These data are indicative of diminished glomerular function. Collectively, the results demonstrate that G3YR mice suffer from nondiabetic mesangial glomerulonephritis, which is associated with chronic, progressive renal failure.
GATA3 is expressed in glomerular mesangial cells.
Given the progressively worsening pathology observed in the glomeruli of G3YR mice, we carefully reexamined the GATA3 expression pattern in the glomerulus. Podocytes and mesangial cells are two of the major cell lineages in the renal glomerulus (27). To delineate which glomerular cell lineage expressed GATA3, we costained kidneys recovered from Gata3g/+ mice with antibodies recognizing CD73 (a mesangial cell marker [28]), synaptopodin (a podocyte marker [29]), or GATA3-GFP expressed from the Gata3g targeted allele. These colocalization studies demonstrated that a large proportion of GATA3-GFP-positive cells coexpressed CD73 (Fig. 6A) while the GFP-labeled cells rarely colocalized with antisynaptopodin (Fig. 6B). These data thus indicate that GATA3 is predominantly expressed in glomerular mesangial cells but not in podocytes.
FIG 6.
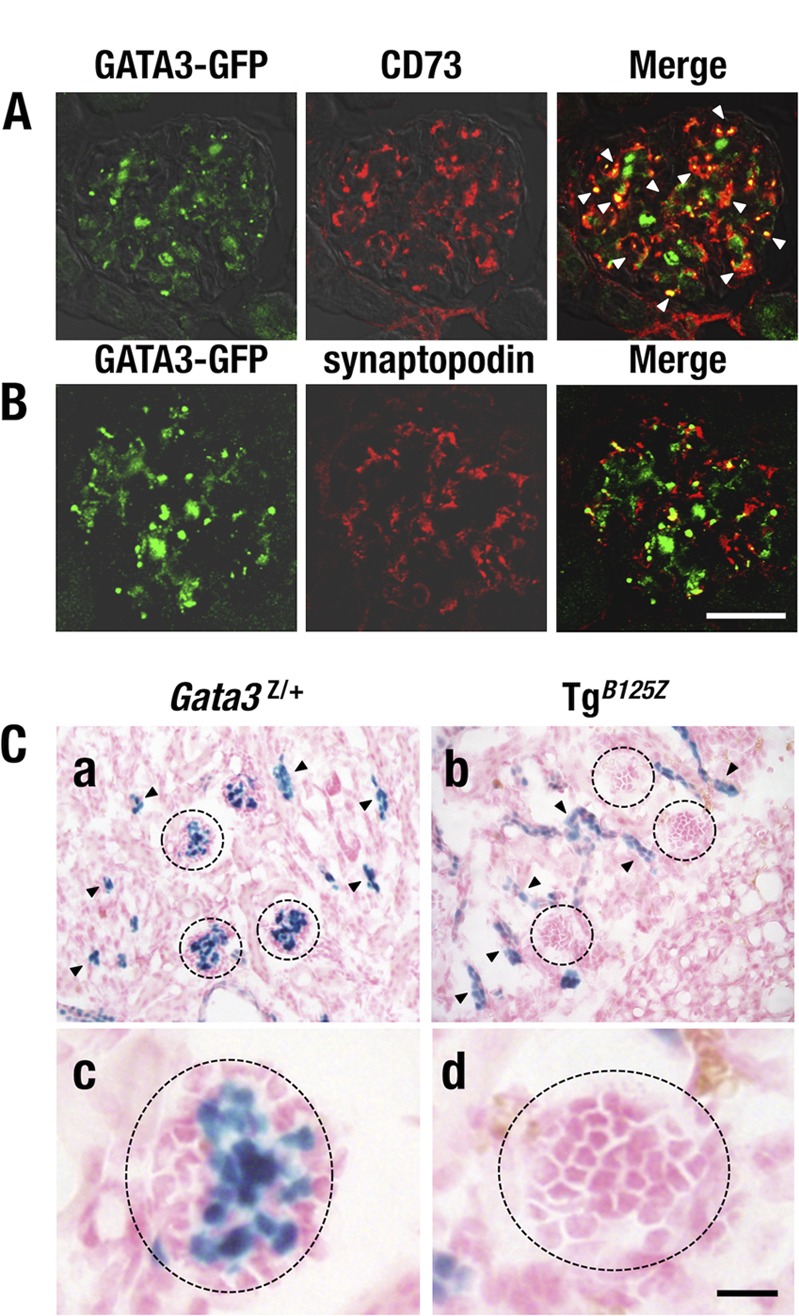
GATA3 expression in the renal glomerulus. (A) GATA3-GFP-positive cells (green) are colocalized with CD73-positive (red) mesangial cells (arrowheads in the merged image). (B) GATA3-GFP (green) and synaptopodin (red) immunoreactivities are rarely colocalized. (C) X-Gal staining of kidney from Gata3Z/+ (left) and TgB125Z (right) mice. Gata3Z/+ mice show lacZ activity-positive cells in the renal tubular cells (arrowheads) and the central region of the glomerulus (circles) (a and c). TgB125Z mice show lacZ-positive cells only in the tubules (arrowheads) (b and d). lacZ activity is largely missing from the glomeruli of TgB125Z mice. Bars, 50 μm.
We next stained the kidneys of Gata3Z/+ knock-in mice with X-Gal to examine the distribution of lacZ-positive cells in the kidney. We found that robust lacZ-positive cells were detected in the central region of the glomerulus, where the glomerular mesangial cells reside (Fig. 6C, panels a and c, circles). The lacZ-positive cells were also detected in the renal tubules of the Gata3Z/+ mice (Fig. 6C, panel a, arrowheads) as reported previously (12). Importantly, TgB125Z mice exhibited lacZ staining only in the tubules (Fig. 6C, panel b, arrowheads), while lacZ activity was largely absent from the glomeruli of TgB125Z mice (Fig. 6C, panels b and d, circles). These results indicate that this transgene might genetically complement the missing GATA3 activity required for tubular development but also that the 662-kb B125 YAC is incapable of directing mesangial-cell-specific Gata3 gene expression. We surmise that even this very large 662-kb region surrounding and containing the Gata3 locus still lacks the required regulatory elements that control glomerular mesangial-cell-specific gene expression.
GATA3 deficiency in mesangial cells alters glomerular gene expression.
We next quantitatively examined the expression pattern of glomerulus- and tubule-related genes. To this end, we separated glomerular and tubular tissues of the kidney from G3YR and wild-type control mice. We used younger (3- to 4-week-old) mice in which minimum progression of glomerulonephritis was in evidence in order to preclude contamination with severely damaged cells. The mice were perfused by intracardiac injection with magnetic beads that become trapped within the glomerular capillaries (24). Kidneys were then digested with dispase, and the glomerulus-enriched fraction was separated using a magnetic particle concentrator (Fig. 7A and B; see Materials and Methods). The glomerular fraction and other kidney cells (the tubular fraction) were separately subjected to RT-qPCR in order to examine the expression of glomerulus- and tubule-related genes. platelet-derived growth factor receptor α (PDGFRα) and PDGFRβ, encoded by Pdgfra and Pdgfrb, respectively, are receptors for platelet-derived growth factor (PDGF), which participates in the development and maintenance of the mesangial cells (30, 31). We found that the level of Pdgfra and Pdgfrb mRNAs was significantly reduced in the glomeruli of the G3YR mice compared with the levels in wild-type mice (Fig. 7C). Podocin and nephrin genes, two podocyte-related genes involved in the formation of filtration slits in the glomerulus (27), were both expressed at lower levels in the G3YR mice than in the wild-type control mice, although the differences did not achieve statistical significance (Fig. 7C). Meanwhile, we found that Avpr2 and Aqp2, two tubular-cell-specific GATA factor target genes (23), are comparably expressed in the tubular cell fraction of the G3YR and wild-type mice (Fig. 7D). These results indicate that expression of mesangial-cell-affiliated genes is most significantly affected in the G3YR kidneys, while expression of tubule-related genes was largely unaffected. This result underscores the possibility that tubular-cell-specific Gata3 complementation by the TgB125-G3 transgene restored the expression of tubular-cell-related genes in G3YR mice.
FIG 7.
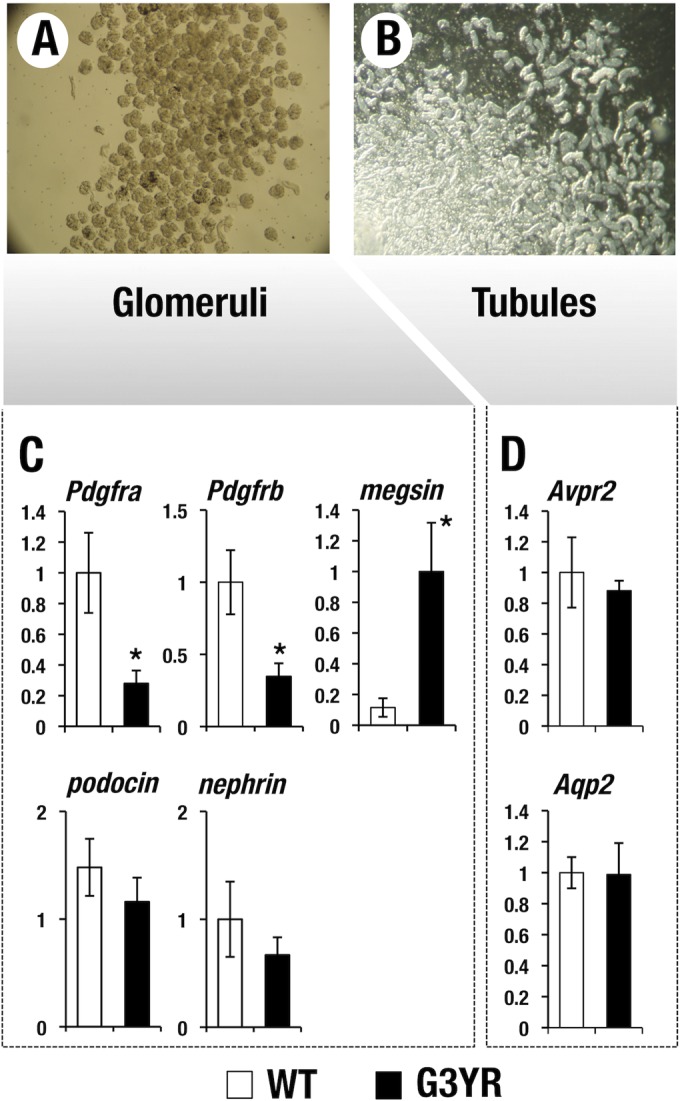
Expression of glomerulus and tubule-related genes in the young G3YR mice. Glomerular (A) and tubular (B) fractions of kidney from wild-type and G3YR (Gata3g/g::TgB125-G3) mice are separately subjected to RT-qPCR analysis. (C) Gene expression signature in the glomerular cells. Expression of Pdgfra and Pdgfrb, a mesangial-cell-specific marker, is significantly decreased in the G3YR mice. Podocin and Nephrin, two representative podocyte markers, are relatively maintained. Megsin, a serine protease inhibitor, is highly induced in the G3YR mice. Statistical significances in differences are indicated (WT, n = 7; G3YR, n = 7; *, P < 0.05; Student's unpaired t test). (D) Gene expression signature in tubular cells. Ae1 and Aqp2, two target genes of the GATA factor, are comparably expressed in the G3YR and wild-type mice.
Megsin, a mesangial-cell-specific serine protease inhibitor, is known to be induced during the progression of glomerular injury and to participate in the pathogenesis of mesangial nephritis (32). Notably, we found that the G3YR mouse kidneys showed significantly increased expression of megsin in the glomerular cell fraction (Fig. 7C). This observation shows that reduced GATA3 activity leads to induction of megsin, which participates in the progression of mesangial nephritis in the G3YR mice, suggesting that GATA3 is a direct or indirect negative regulator of megsin mRNA accumulation. Collectively, these results demonstrate that GATA3 is highly expressed in glomerular mesangial cells and plays a crucial role in their maintenance and thereby contributes to the prevention of glomerular nephritis.
DISCUSSION
In the present study, we demonstrated that a fusion protein generated by inserting a GATA3 cDNA ligated in frame to GFP into the Gata3 gene results in the generation of hypomorphic mutant mice. This hypomorphic activity of Gata3g allele could be attributable primarily to the lower abundance of GATA3-GFP protein. Additionally, the altered transcriptional activity of GATA3-GFP protein might also contribute to the hypomorphic activity of the GATA3-GFP allele. Given the relatively large molecular mass of GFP, GFP fusion to the C-terminal may affect the activity of GATA3.
We demonstrated that homozygous mutant hypomorphs survive longer than do Gata3-null mutant mice and yet still expire at the neonatal stage. We proposed that this novel execution time for neonatal lethality due to the homozygous Gata3g/g mutant allele was due to severe kidney hypotrophy and craniofacial defects. In support of this hypothesis, the initial perinatal lethality was successfully rescued by crossing with a transgenic mouse line harboring a YAC containing 662 kb both surrounding and including the Gata3 locus. This YAC was capable of directing GATA3 expression in the pharyngeal arch and the ureteric bud-derived renal tubules but lacks the regulatory activity for renal glomerular mesangial-cell-specific Gata3 expression (summarized in Fig. 8). As a secondary possible explanation, the YAC-directed transgenic GATA3 expression might be attenuated by position effect variegation of transgene integration. Consequently, the transgenic GATA3 expression might fail to reach the threshold activity required for maintenance of mesangial cells, albeit other parts of kidney undergo normal development. If this hypothesis is correct, our results may suggest that a high level of GATA3 activity is a prime requisite for maintenance of mesangial cells. Alternatively, the YAC transgene integration site could be at a genomic position that somehow suppressed mesangial gene expression from the (fully rescuing) YAC.
FIG 8.
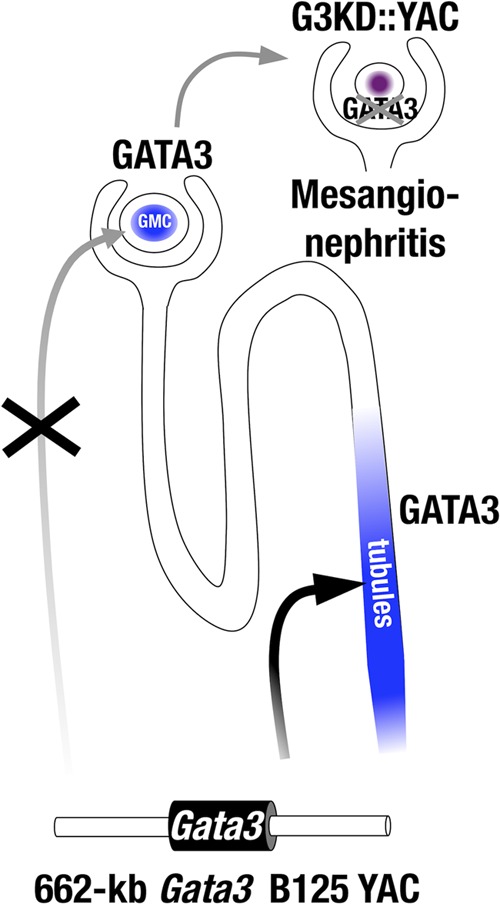
Mesangionephritis in the G3YR mice. Gata3 is expressed in both tubular cells and glomerular mesangial cells (GMC). The 625-kb Gata3 YAC directs Gata3 expression in tubular cells but not mesangial cells. G3YR (Gata3g/g::TgB125-G3) mice show mesangionephritis because the B125 Gata3 YAC fails to complement GATA3 in mesangial cells.
We previously reported the activity of a urogenital primordium-specific enhancer, located 113 kb 5′ to the Gata3 structural gene (12). Transgenic GATA3 expression directed by this single enhancer successfully rescues an embryonic kidney developmental defect detected in Gata3-null embryos (12). Meanwhile, it is known that the newborn kidney remains structurally and functionally primitive and that a substantial part of nephron function is achieved during postnatal development (33). Importantly, glomerular mesangial cells retain self-renewal activity and replace themselves upon cellular insults even after birth (27). We presume that additional regulatory activity would be needed to direct GATA3 expression specifically in mesangial cells to execute their postnatal differentiation and maintenance.
Due to diminished GATA3 activity in the glomerular mesangial cells, the G3YR mice exhibited severe and specific degenerative changes and subsequently developed progressive renal failure. These observations suggest that a precise level of GATA3 activity must be stringently regulated for maintenance of mesangial cells and for prevention from glomerular disease. It is well established that PDGF secreted by podocytes and endothelial cells transmit growth signals through binding to the PDGF receptors (α and β) expressed on the mesangial cell surface to promote their differentiation and maintenance (31). It was reported that PDGFRβ-deficient mice exhibit ballooning of glomerular capillary tufts (34), which is remarkably reminiscent of the glomerular abnormality displayed by G3YR rescued mice. Considering that the glomerular cells of the G3YR mice also had diminished expression of PDGFRα and -β, we surmise that the GATA3-PDGFR regulatory axis may play a crucial role in the differentiation and maintenance of mesangial cells.
Embryonic urogenital development begins when a ureteric bud (UB) sprouts from the posterior mesonephric tubules and then branches into the adjacent metanephric mesenchyme (MM). Reciprocal inductive signals between these two mesodermal derivatives govern the subsequent kidney developmental program (35). Gata2 and Gata3 are coexpressed in ureteric bud epithelial cells of the mouse embryo (36, 37), and we demonstrated previously that GATA2 plays an essential role in ureter-bladder junctional development (37, 38). Perturbation of this process leads to hydronephrosis and megaureter in the Gata2 hypomorphic mutant neonatal mice (37, 39). The Gata3 hypomorphic mutant neonate mice that were generated in this study exhibited severely hypoplastic kidneys, whereas there was scant evidence for hydronephrosis or megaureter (data not shown). These results indicate that GATA2 and GATA3, despite their similar, often overlapping, expression profile in the urogenital system, exert different functions in early urogenital development. Detailed analysis of the distinct versus redundant function of these two urogenital GATA factors is of particular importance, given that these two genes account for distinct urogenital anomalies in inherited human diseases (15, 40, 41).
During definitive kidney development, the UB gives rise to the collecting ducts and the ureter while MM differentiates into glomerulus and proximal and distal tubules (35, 42, 43). During embryonic renal development, GATA3 is expressed predominantly in the UB tissues and not in the MM (12, 36). Subsequently, GATA3 expression is maintained in the UB-derived adult renal tubular collecting-duct cells (23). The developmental origin of mesangial cells appears to be distinct from that of the UB, because recent lineage tracing studies with FoxD1-CreERT2 mice have revealed that mesangial cells are derived from the FoxD1-expressing stroma cells, neither UB nor MM (44). Given these data, robust GATA3 expression in the glomerular mesangial cells of the adult kidney cannot be explained by classical ontogeny, posing a conundrum that we currently plan to investigate by lineage tracing.
A series of studies have reported that a fraction of glomerular mesangial cells are derived from bone marrow hematopoietic cells (45, 46). Bone marrow transplantation into nephritis model mice facilitates the replacement of mesangial cells with normal bone marrow-derived cells (45). In support of this model, it is also known that mesangial cells express hematopoietic cell surface immune phenotypes. For example, rat mesangial cells express Thy1.1 (thymocyte differentiation antigen 1) as a specific marker (47), and administration of anti-Thy1.1 antisera evokes mesangial proliferative glomerulonephritis in rat (47). These results raise the possibility that mesangial cells retain hematopoietic phenotypes. GATA3 is thought to be a master regulator of T-lymphocyte differentiation and is specifically expressed in thymocyte lineage cells in the adult hematopoietic system (reviewed in reference 48). Robust expression of GATA3 in thymocytes and glomerular mesangial cells may imply an ontogenic relationship between thymocytes and the bone marrow-derived mesangial cells.
Tissue-specific cis elements for Gata3 gene are distributed over an extensive area surrounding and within the Gata3 gene locus. Indeed, a thymocyte-specific regulatory element for Gata3 gene has been identified beyond the boundaries described by the B125 YAC, around 280 kb 3′ to the Gata3 structural gene (49, 50). Discovery of the missing mesangial-cell-specific cis-regulatory element that presumably lies outside the domain described by the B125 YAC would be of significant interest for future study. A potential common regulatory mechanism for Gata3 in the two lineages of bone marrow-derived (T lymphocyte and mesangial) cells would constitute an interesting hypothesis to further explore.
In summary, the present study demonstrates a previously unrevealed function for transcription factor GATA3 in glomerular mesangial cells. Further analysis to comprehensively address the set of GATA3 target genes that govern renal glomerular mesangial cell differentiation and elucidation of their precise physiological roles should significantly expand our understanding of this newly discovered function of GATA3 in the kidney. Furthermore, we anticipate that the G3YR mice may serve as a faithful model to elucidate the pathogenic mechanisms leading to glomerular nephritis, thereby potentially serving a role through which we may be able to develop therapeutic strategies to treat this highly prevalent kidney disease.
ACKNOWLEDGMENTS
We thank Cristina Cebrian, Michito Hamada, Tomokazu Souma, and Mikiko Suzuki for discussion and critical review of this paper. We thank the Microscopy and Imaging Analysis Laboratory for assistance with electron microscopy and the Transgenic Animal Model Core of the University of Michigan. We also thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for its technical support.
This study was supported in part by the Japan Society for the Promotion of Science (JSPS) (KAKENHI 26670463, 26111002, and 15H02507 to M.Y. and 16H05147 to T.M.), AMED-CREST (to M.Y.), the Naito Foundation, Mitsubishi Foundation, and Takeda Science Foundation (to M.Y.), the Kobayashi Foundation for Cancer Research (to T.M.), and NIH R01 GM28896 (to J.D.E.).
REFERENCES
- 1.Uhlenhaut NH, Treiser M. 2008. Transcriptional regulators in kidney disease: gatekeepers of renal homeostasis. Trends Genet 24:361–371. doi: 10.1016/j.tig.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. 1990. Activity and tissue-specific expression of the transcription factor NF-E1 [GATA] multigene family. Genes Dev 4:1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- 3.Martin DI, Orkin SH. 1990. Transcriptional activation and DNA-binding by the erythroid factor [GATA-1]. Genes Dev 4:1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- 4.Merika M, Orkin SH. 1993. DNA-binding specificity of GATA family transcription factors. Mol Cell Biol 13:3999–4010. doi: 10.1128/MCB.13.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko LJ, Engel JD. 1993. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol 13:4011–4022. doi: 10.1128/MCB.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George KM, Leonard MW, Roth MW, Liew KW, Kioussis D, Grosveld F, Engel JD. 1994. Embryonic expression and cloning of the murine GATA-3 gene. Development 120:2673–2686. [DOI] [PubMed] [Google Scholar]
- 7.Lieuw KW, Li G, Zhou Y, Grosveld F, Engel JD. 1997. Temporal and spatial control of murine GATA-3 transcription by promotor-proximal regulatory elements. Dev Biol 188:1–16. doi: 10.1006/dbio.1997.8575. [DOI] [PubMed] [Google Scholar]
- 8.Lakshmanan G, Liew KH, Grosveld F, Engel JD. 1998. Partial rescue of GATA-3 by yeast artificial chromosome transgene. Dev Biol 204:451–463. doi: 10.1006/dbio.1998.8991. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmanan G, Liew KH, Lim KC, Gu Y, Grosveld F, Engel JD, Karis A. 1999. Localization of distant urogenital system-, central nervous system-, and endocardium-specific transcriptional regulatory elements in the GATA-3 locus. Mol Cell Biol 19:1558–1568. doi: 10.1128/MCB.19.2.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. 1995. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and fetal liver haematopoiesis. Nat Genet 11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 11.Lim K-C, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. 2000. GATA3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet 25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa SL, Moriguchi T, Rao A, Kuroha T, Engel JD, Lim KC. 2007. Dosage-dependent rescue of definitive nephrogenesis by a distant Gata3 enhancer. Dev Biol 301:568–577. doi: 10.1016/j.ydbio.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriguchi T, Nakano T, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim K-C, Engel JD. 2006. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 133:3871–3881. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- 14.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. 2000. GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 15.Van Esch H, Devriendt K. 2001. Transcription factor GATA3 and the human HDR syndrome. Cell Mol Life Sci 58:1296–1300. doi: 10.1007/PL00000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chenouard A, Isidor B, Allain-Launay E, Moreau A, Le Bideau M, Roussey G. 2013. Renal phenotypic variability in HDR syndrome: glomerular nephropathy as a novel finding. Eur J Pediatr 172:107–110. doi: 10.1007/s00431-012-1845-y. [DOI] [PubMed] [Google Scholar]
- 17.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A 90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. 2000. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 19.van Doorninck JH, Van der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, Rutteman M, Grosveld F, De Zeeuw CI. 1999. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci 19(12):RC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takai J, Moriguchi T, Suzuki M, Yu L, Ohneda K, Yamamoto M. 2013. The Gata1 5′ region harbors distinct cis-regulatory modules that direct gene activation in erythroid cells and gene inactivation in HSCs. Blood 122:3450–3460. doi: 10.1182/blood-2013-01-476911. [DOI] [PubMed] [Google Scholar]
- 21.Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S. 2006. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol 26:5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda A, Moriguchi T, Hamada M, Kusakabe M, Fujioka Y, Nakano T, Yoh K, Lim KC, Engel JD, Takahashi S. 2009. Transcription factor GATA-3 is essential for lens development. Dev Dyn 238:2280–2291. doi: 10.1002/dvdy.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L, Moriguchi T, Souma T, Takai J, Satoh H, Morito N, Engel JD, Yamamoto M. 2014. GATA2 regulates body water homeostasis through maintaining aquaporin 2 expression in renal collecting ducts. Mol Cell Biol 34:1929–1941. doi: 10.1128/MCB.01659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. 2002. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, Engel JD. 2009. GATA-3 is required for early T lineage progenitor development. J Exp Med 206:2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devarajan P. 2011. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr 23:194–200. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quaggin SE, Kreidberg JA. 2008. Development of the renal glomerulus: good neighbors and good fences. Development 135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- 28.Blume C, Felix A, Shushakova N, Gueler F, Falk CS, Haller H, Schrader J. 2012. Autoimmunity in CD73/Ecto-5′-nucleotidase deficient mice induces renal injury. PLoS One 7:e37100. doi: 10.1371/journal.pone.0037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. 1997. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. 1998. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125:3313–3322. [DOI] [PubMed] [Google Scholar]
- 31.Floege J, Eitner F, Alpers CE. 2008. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol 19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 32.Inagi R, Nangaku M, Usuda N, Shimizu A, Onogi H, Izuhara Y, Nakazato K, Ueda Y, Oishi H, Takahashi S, Yamamoto M, Suzuki D, Kurokawa K, van Ypersele de Strihou C, Miyata T. 2005. Novel serpinopathy in rat kidney and pancreas induced by overexpression of megsin. J Am Soc Nephrol 16:1339–1349. doi: 10.1681/ASN.2004070600. [DOI] [PubMed] [Google Scholar]
- 33.Little MH, McMahon AP. 2012. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4(5):pii:a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soriano P. 1994. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 35.Dressler G. 2002. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol 12:390–395. doi: 10.1016/S0962-8924(02)02334-6. [DOI] [PubMed] [Google Scholar]
- 36.Khandekar M, Suzuki N, Lewton J, Yamamoto M, Engel JD. 2004. Multiple, distant Gata2 enhancers specify temporally and tissue-specific patterning in the developing urogenital system. Mol Cell Biol 24:10263–10276. doi: 10.1128/MCB.24.23.10263-10276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ainoya K, Moriguchi T, Ohmori S, Souma T, Takai J, Morita M, Chandler KJ, Mortlock DP, Shimizu R, Engel JD, Lim KC, Yamamoto M. 2012. UG4 enhancer-driven GATA-2 and bone morphogenetic protein 4 complementation remedies the CAKUT phenotype in Gata2 hypomorphic mutant mice. Mol Cell Biol 32:2312–2322. doi: 10.1128/MCB.06699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Lim KC, Onodera K, Takahashi S, Ohta J, Minegishi N, Tsai FY, Orkin SH, Yamamoto M, Engel JD. 1998. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J 17:6689–6700. doi: 10.1093/emboj/17.22.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoshino T, Shimizu R, Ohmori S, Nagano M, Pan X, Ohneda O, Khandekar M, Yamamoto M, Lim KC, Engel JD. 2008. Reduced BMP4 abundance in Gata2 hypomorphic mutant mice result in uropathies resembling human CAKUT. Genes Cells 13:159–170. doi: 10.1111/j.1365-2443.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 40.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, Dafou D, Kilo T, Smithson S, Lunt P, Murday VA, Hodgson S, Keenan R, Pilz DT, Martinez-Corral I, Makinen T, Mortimer PS, Jeffery S, Trembath RC, Mansour S. 2011. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 41.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. 2014. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riccio P, Cebrian C, Zong H, Hippenmeyer S, Costantini F. 2016. Ret and Etv4 promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol 14:e1002382. doi: 10.1371/journal.pbio.1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cebrian C, Asai N, D'Agati V, Costantini F. 2014. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep 7:127–137. doi: 10.1016/j.celrep.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP. 2014. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep 3:650–662. doi: 10.1016/j.stemcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuya M, Drake CJ, Fleming PA, Reilly CM, Zeng H, Hill WD, Martin-Studdard A, Hess DC, Ogawa M. 2003. Hematopoietic origin of glomerular mesangial cells. Blood 101:2215–2218. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 46.Ito T, Suzuki A, Imai E, Okabe M, Hori M. 2001. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol 12:2625–2635. [DOI] [PubMed] [Google Scholar]
- 47.Schlöndorff D, Banas B. 2009. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 48.Hosoya T, Maillard I, Engel JD. 2010. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev 238:110–125. doi: 10.1111/j.1600-065X.2010.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosoya-Ohmura S, Lin YH, Herrmann M, Kuroha T, Rao A, Moriguchi T, Lim KC, Hosoya T, Engel JD. 2011. An NK and T cell enhancer lies 280 kilobase pairs 3′ to the gata3 structural gene. Mol Cell Biol 31:1894–1904. doi: 10.1128/MCB.05065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohmura S, Mizuno S, Oishi H, Ku CJ, Hermann M, Hosoya T, Takahashi S, Engel JD. 2016. Lineage-affiliated transcription factors bind the Gata3 Tce1 enhancer to mediate lineage-specific programs. J Clin Invest 126:865–878. doi: 10.1172/JCI83894. [DOI] [PMC free article] [PubMed] [Google Scholar]