Abstract
Ultrasound is well known as a low-cost, radiation-free and effective imaging technique to guide percutaneous procedures. The lower limb muscles represent a good target to perform such procedures under ultrasound guidance, thus allowing for clear and precise visualization of the needle during the whole procedure. The knowledge of guidelines and technical aspects is mandatory to act in the most safe and accurate way on target tissues that can be as small as a few millimetres. This review will focus above the local treatments of traumatic lower limb muscle injuries described in literature, focusing on new and promising approaches, such as platelet-rich plasma treatment of muscle tears in athletes. For each procedure, a brief how-to-do practical guide will be provided, emphasizing precautions and tricks based on day-by-day experience that may help to improve the outcome of percutaneous ultrasound-guided procedures around the lower limb muscles.
INTRODUCTION
Over the past decade, with recent technological advancements, interventional musculoskeletal (MSK) image-guided procedures have been increasingly spread in clinical practice. Dynamic high-resolution ultrasonography has emerged as the referential imaging modality to guide infiltrative manoeuvres in many articular, tendon and muscle pathologies. Ultrasound is a quick, portable and cost-effective method that offers the possibility of a real-time visualization of both the target structure and the needle during the entire procedure, allowing the operator to reach the precise injection site (high drug-deposition accuracy). It allows for careful avoidance of crucial structures such as neurovascular bundles, thus reducing the risk of complications.1–6 Moreover, the lack of ionizing radiation makes ultrasound-guided injection techniques safe and repeatable. These various advantages have contributed to expansion in the routine application of ultrasound guidance in the field of MSK interventional procedures; indeed, ultrasound-guided percutaneous treatments have gradually replaced the traditional blind manoeuvres in respect to which they offer a better patient outcome in terms of pain reduction during and after the injection or aspiration, greater efficacy and improvement of post-procedural articular or muscular functionality.7–9
Currently, a large number of percutaneous minimally invasive treatments can be performed under ultrasound guidance: aspiration of joint fluid collections, drug injection in the joints, tendon sheaths, bursae and perineural soft tissues, lavage of tendon calcifications, tendon dry needling, injection of regenerative agents in injured tendons and muscles, aspiration of muscular haematomas and biopsies of soft-tissue masses.
One of the main MSK clinical fields in which these ultrasound-guided infiltrative procedures have created interest is sports medicine. Sport physicians are familiar with tendon and especially muscle injuries; muscular contusions and strains represent the most common findings, accounting for approximately 10–55% of all sport-related lesions.10,11 In particular, the hamstrings, rectus femoris, gracilis and medial head of the gastrocnemius are typically involved.12,13
Muscle injuries have an important impact especially on professional athletes because they are the most frequent cause of days lost from training and competition.14 Thus, the primary aim of the medical staff of professional clubs is to promote a full recovery with a rapid return to play of the injured athlete. In this setting, besides the traditional conservative treatments (rest, ice, compression and elevation protocols, non-steroidal anti-inflammatory drugs, physical therapy and functional rehabilitation), in recent years, with current biotechnological innovations, additional therapeutic ultrasound-guided options have been developed.15,16 A number of biological factors such as the platelet-rich plasma (PRP) and stem cells can be directly injected under ultrasound guidance at the site of the lesion to facilitate muscle regeneration and minimize the formation of a dense scar tissue. The goal of these novel agents is to improve the healing process of the injured muscle with a return to pre-injury muscle functionality as soon as possible. Another useful application of ultrasound-guided procedures is intermuscular or intramuscular haematomas aspiration, which may be performed in selected cases.
Our review focuses on the most widely accepted interventional ultrasound-guided procedures employed to treat skeletal muscle sport-related injuries; in particular, platelet concentrate injection and evacuation of muscular haematomas, with few considerations about new perspectives. We will also discuss some useful practical aspect required to perform safe and accurate interventional procedures.
Basic principles of ultrasound-guided interventional procedures
The following considerations should be kept in mind prior to any procedure.
Basic clinical information
A preliminary accurate collection about the patient's medical history is recommended. In particular, referring to traumatic muscle injuries, it is important to ask the patient when the trauma happened and elucidate the dynamics of the trauma. Past medical history, family medical history and personal/social medical history could be helpful to correctly evaluate the patient. Further considerations should be assessed about possible history of drug allergies, consumption of anticoagulant/antiplatelet drugs or the presence of blood-thinning pathologies that could cause severe bleeding after the procedure.
Contraindications, complications and informed consent
Ultrasound-guided interventional MSK manoeuvres are minimally invasive and associated with a low complication rate; however, the operator must give details about the contraindications and potential complications related to the planned procedure to the patient for obtaining verbal and written informed consent.
Patient positioning
The patient must be placed on the examination table in a comfortable position in order to avoid any potential movements during the procedure.
Antisepsis
All ultrasound-guided interventional manoeuvres require an aseptic setting in order to avoid any risk of contamination and secondary infectious. Antisepsis includes operator sterility, probe and skin antisepsis. Many published data show that these procedures are secure in order to prevent secondary infections.1,17,18
Probe selection
The choice of the probe is based on the depth of the target structure. During MSK interventional procedures, linear phased array probes (5–12 MHz up to 18 MHz) are usually preferred because they allow a high-resolution visualization of superficial structures. Deep muscle injuries, athletes with large muscular mass or obese patients may require the use of a convex probe (3–5 MHz).
Interventional equipment
Needle choice should be based mainly on the depth of the anatomic structure to treat. Also the density concentration of the drug to inject and the expected viscosity of the fluid to aspirate must also be considered. Spinal needles are used for deep locations, athletes with large muscular mass or obese patients. Frequently, 18–20 G needles are used for many interventional procedures; dense blood collections may require larger (14–16 G) needles. Single-use syringes, drainage tubes and catheters are preferred. The choice of syringe size strictly depends on the amount of fluid to inject or drain.
Ultrasound-guided interventional procedure
A preliminary ultrasound evaluation is recommended in order to identify the most reliable procedure setting and to confirm the expected findings. The interventional manoeuvres can be performed using two main approaches: coaxial and lateral. In the lateral approach, the needle is placed and inserted parallel to the ultrasound beam, on the short side of the probe; it has the advantage of excellent visibility of the needle during the entire procedure, allowing the operator to reach the precise site of injection/aspiration. The coaxial approach is burdened by reduced needle visibility; it can be used when the space around the target is greatly restricted.
Ultrasound-guided aspiration procedures
As mentioned previously, contusive and/or strain traumas account for the majority of sport-related injuries. Usually, they are managed with a conservative approach and heal spontaneously after variable amounts of time with complete restoring of the tissue.10,19 In some cases, the natural course of such injuries do not completely take place and some complications may occur. These complications can induce many clinical manifestations which variably modify patient outcome and management during rehabilitation period: chronic haematomas and muscle atrophy may delay the healing process, a predominant scar tissue may increase the risk of injuries recurrence and myositis ossificans may simulate a soft-tissue tumour, in particular when clinical data are unclear. Ultrasound-guided percutaneous procedures play an important role in some of those cases.20,21
Haematomas
Muscle haematomas represent the direct consequence after a muscular strain or contusion. They could be intramuscular when confined within a single muscle, among its fibres, or intermuscular when located between two or more muscles, along fascial planes.22 Intramuscular haematomas are less common; they may decrease in dimension over time but are less likely to resolve spontaneously than intermuscular haematomas.20,22 A chronic haematoma is frequently seen in the triceps surae muscle in tennis leg setting, as a consequence of a tear involving the aponeurosis between the soleus and gastrocnemius muscles;23,24 another common location is the vastus intermedius muscle, which is particularly prone to developing haematomas after contusive traumas owing to its location just over the femur.25
At ultrasound evaluation, initially haematoma may demonstrate variable appearance with muscle swelling and oedema, ranging from anechoic or hypoechoic to hyperechoic. As the organization progresses in the subsequent 2–3 days, the walls become more evident and the amount of fluid becomes progressively hypoechoic or anechoic: this is the ideal time for ultrasound examination to detect haematomas in case of low-grade muscular strain.10,19 Following weeks after injury, the amount of fluid progressively decreases and its echogenicity increases; meanwhile, its walls thicken towards the centre until the fluid closes. Many times, the fluid component does not appear as homogeneously hypo-anechoic but may present increased central echogenicity and fluid/debris levels.23 Usually chronic muscular haematomas take many weeks to resolve but sometimes do not resolve spontaneously, resulting in significant delay in the return to sport practice and predisposing to possible complications such as partial calcification, cyst formation, myositis ossificans, neural compression and even expansion, causing compartment syndrome.19–23,26 In those cases in which chronic haematomas do not resolve in time, ultrasound-guided percutaneous aspiration represents the first-line approach in order to drain the fluid collected and promote the healing process.6 However, consider the risk of infection during procedure, in particular in incompletely aspirated collections of blood. Aspiration may be used if there is severe pain, in particular in case of significant intermuscular haematomas, or to promote early healing (i.e. for elite athletes). Further, ultrasound is useful to identify features that could increase procedure difficulty (i.e. extensive loculation of the haematoma during organization).20
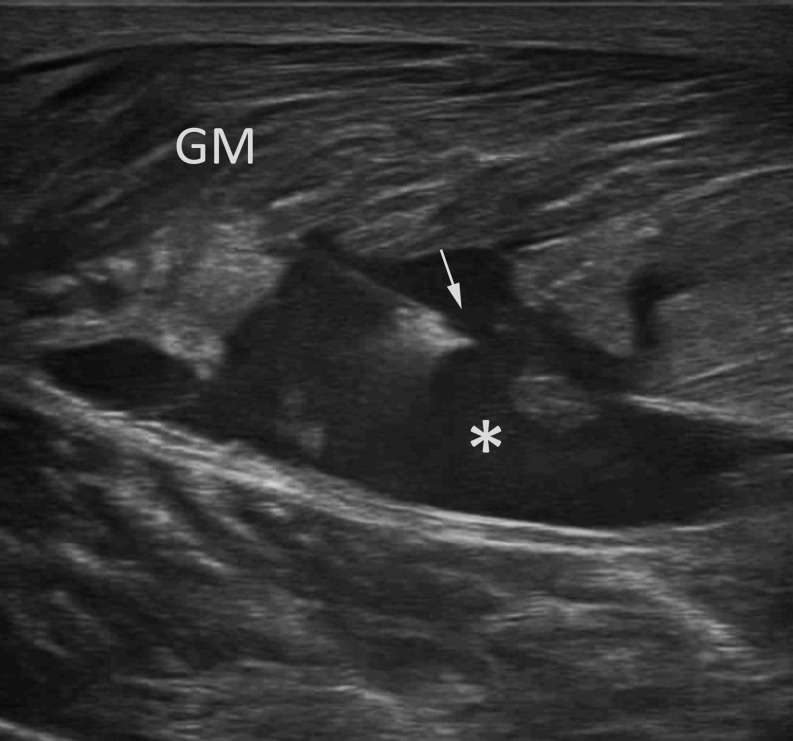
By definition, aspiration refers to the use of a catheter or needle to evacuate fluid collection with immediate post-procedural removal of the needle or catheter (without continuous drainage).27 The procedure cannot be performed until most of the haematoma has sufficiently liquefied; so, the ideal time is between 1 and 2 weeks after injury. This appearance can be assessed by repeated ultrasound scans of the fluid collection, evaluating the internal echotexture and haematoma compressibility under pressure of the transducer. Ultrasound-guided needle aspiration of the haematoma can easily be performed with a freehand technique, until complete evacuation; a 10 ml syringe and 18–20-G standard needle are generally appropriate; pressing the tissues around the collection in order to increase the amount of fluid to be aspirated may be a helpful manoeuvre (Figure 1).
Figure 1.
Ultrasound-guided aspiration of a large gemellus medialis (GM) haematoma (*) using a 20-G spinal needle (arrow).
A thigh elastic bandage must be performed after the procedure in order to avoid the possibility of recurrence of the fluid collection and a period of rest of about 24–48 h is indicated.28 After 1 week of the procedure, it is useful to review the outcome of the procedure with a ultrasound follow-up: if the haematoma has recurred, it is possible to perform the aspiration again. Usually haematoma aspirations do not need any subsequent drug injection; anyway, a small amount of corticosteroid can be injected at the same site at the end of the procedure,27–29 in particular in case of recurrence of the haematoma and/or only partial aspiration of the fluid collection.
Cysts
Sometimes, after muscular traumas, intermuscular and/or intramuscular cysts may be detected at follow-up. They represent the residue of a local pre-existing haematoma, in most cases significant in terms of dimension. They can stand for months and their most common location is the calf, but some cases are reported also in the rectus femoris, semi-membranosus and semi-tendinosus muscles. Usually cysts have an elongated shape according to the three-dimensional disposition of the muscular fibres and the fascial planes in which they are located. At ultrasound examination, they appear as well-defined anechoic lesions with posterior acoustic enhancement.30 Although imaging features of cysts are usually pathognomonic, it is very important to relate such ultrasound findings to the history of traumatic injuries: always consider the differential diagnosis list which includes the synovial cyst or articular ganglia and bursae; as for chronic haematomas, in rare cases, some soft-tissue tumours (i.e. myxoma, sarcoma) may present cystic appearance and, in the absence of a history of trauma, it is mandatory to investigate the nature of such lesion with further imaging modalities (i.e. MRI with contrast material), in particular if the lesion presents wall thickening or internal complexity.31,32 Ultrasound-guided needle aspiration of the cyst can easily be performed with a freehand technique and a 18–20-G needle until complete evacuation.
Morel-Lavallé
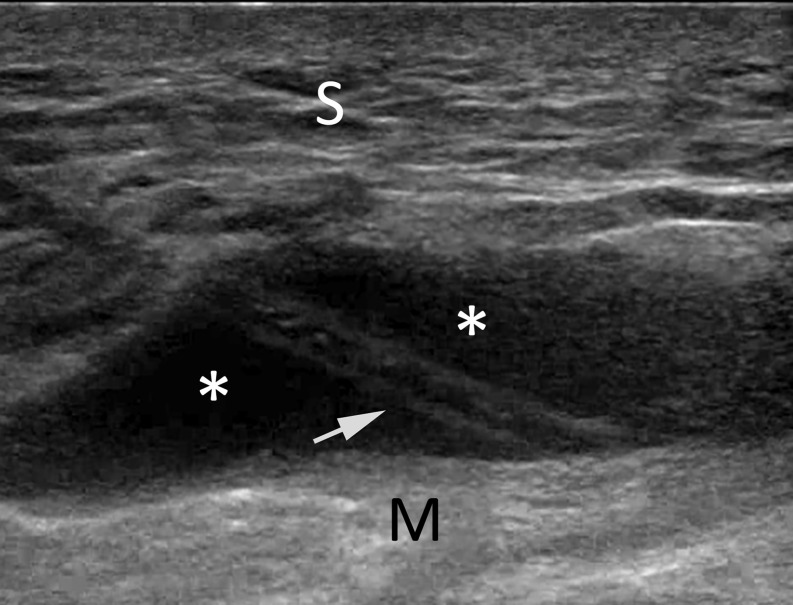
Morel-Lavallée lesion is a post-traumatic haemolymphatic collection occurring after a shearing injury with disruption of interfascial planes between the subcutaneous fat tissue and deep fascia and muscle. Such lesions have different possible presentations ranging from seroma to chronic organizing and/or expanding haematoma.33 Their most common location is the thigh at the level of the greater trochanter but may also be present around the knee;34 other possible locations are the calf and trunk. When not treated in acute setting, Morel-Lavallée lesion may develop an inflammatory reaction and, subsequently, a fibrous capsule, which contributes to the self-perpetuation and eventual slow growth of such process. Various treatment options are reported and include conservative approaches such as compression banding, aspiration, surgical drainage, incision and evacuation with or without sclerosing agents and radical surgery.35 When the lesion is not associated with bone fractures and/or infections and the skin is viable, percutaneous aspiration under ultrasound guidance is possible; considering the significant dimensions of this kind of lesions, it is appropriate to use a 16–18-G needle or cannula, coupled with a 10–20 ml syringe (Figure 2). It is reported that a high amount of fluid and the presence of a fibrous capsule may increase the possibility of recurrence of the lesion. Many case reports reported limited success with percutaneous aspiration, often requiring repeated aspirations, with time to healing ranging from 4 weeks to 10 months. Some authors35,36 proposed useful algorithms to manage Morel-Lavallée lesions; in particular, the Mayo clinic experience36 proposed operative intervention in the presence of fluid collection of >50 ml.
Figure 2.
Drainage of a well-defined hypo-anechoic fluid collection (Morel-Lavallee lesion) (*), superficial to the linear echogenic deep fascia (M). S, subcutaneous fat; 18-G cannula, arrow.
PLATELET-RICH PLASMA INJECTION
By definition, the term PRP describes a preparation obtained from peripheral blood with enrichment of the platelet fraction.37,38 It has been used for decades to accelerate tissue repair during surgery and to treat large or non-healing tears, and since then it has progressively gained a role as an agent of tissue regeneration.39,40 In 2007, the term and definition of PRP was introduced in Pubmed as a medical subject heading to be used for indexing scientific articles, suggesting its application in a variety of clinical settings: focusing on sports medicine and orthopaedics fields; it has been proposed for osteoarthritis, tendinopathies, muscle and tendon injuries, ligamentous injuries, peripheral neuropaties and plantar fascitiis.40,41
The rationale of using PRP resides in the physiological process of healing after tissue damage. It is a complex regenerative process, which comprises immune response [leukocyte infiltration, cellular apoptosis, release of cytokines and growth factors (GFs) and macrophage polarization], angiogenesis, stem cell activation, nerve repair and mechanical stimuli. All these biological responses are coordinated and regulated through the secretion of multipleGFs and cytokines, released by satellite cells, macrophages, platelets, endothelial cells and myofibroblasts.42–46 The entire process usually take place in at least 1 month and leads damaged tissue through an inflammatory phase, reparative phase and remodelling phase to the complete reconstitution of the tissue. The healing process is achieved through the regeneration of muscle fibres and the formation of a fibrotic tissue; the balance between these two mechanisms (acting simultaneously and competitively) represents a crucial point and is at the centre of many studies.
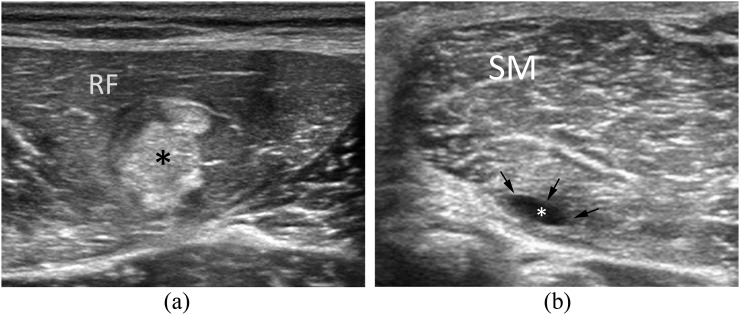
The rationale behind PRP use is to stimulate platelets to release these factors and promote myogenesis, angiogenesis and innervations, and modulate immune response and fibrogenesis to enable functional muscle healing in order to hasten muscular fibres repair and reduce pain during rehabilitation.43,47,48 In our experience, PRP-treated injuries may present different patterns of healing process (seroma, fibrous scar and regenerative tissue) depending on the severity of the injury, on the subjective inflammatory response of the patient and on the rehabilitative mobilization protocol (Figure 3a,b).
Figure 3.
Muscle tear outcome patterns at ultrasound follow-up of patients 20 days after treatment with platelet-rich plasma. (3a) Wide hyperechoic fibrotic tissue (*) affecting the lateral aspect of the rectur femoris (RF) muscle. (b) Deep pseudocystic lesion affecting the semi-membranosus (SM) muscle. In this case, the treated tear (*) is healed with a residual seroma (arrows).
The mechanical stimuli sought and promoted during rehabilitation are essential to the optimal outcome of the PRP usage for injury recovery (myogenesis stimulation, correct alignment of new fibres and proper innervation promotion).
Preparations of PRP may be obtained from the transfusion medicine service of the hospital or prepared using disposable kits. Predetermined PRP concentrations and almost no risk of contamination represent the advantages of using disposable kits, with the higher cost to be taken into consideration.
Many different preparations of PRP are available which differ among themselves in the platelet concentration, release rate of GFs, white and red cell concentration, centrifugation and activation method.37,49–52
In 2009, Dohan et al53 proposed the present nomenclature and classification of platelet-rich preparations based on the cell content and the fibrin architecture: pure or leukocyte-poor PRP, referred to as P-PRP, and leukocyte PRP, termed L-PRP, which are injectable PRP preparations used in sport medicine as liquid solutions or activated gel forms.54 Reported data indicate that L-PRP contains 5-fold to 8-fold more platelets and more leukocytes than peripheral blood, pure or leukocyte-poor PRP has a lower increase in platelet count (1.5- and 2.5-fold above baseline) and leukocytes are absent.41,55
In the following years, other authors reported lists of different preparations commercially available: Mishra et al56 proposed a classification only for sports medicine applications and taking into consideration platelet and leukocyte concentrations. Another system called PAW (platelets, activation, white cells) focuses on the platelet quantity, the activation modality of the platelets and the presence of leukocytes.57
PRP could be prepared also with centrifugation at transfusional centre. This preparation method gives the possibility to match the needed PRP amount and concentration with the various clinical settings; it has lower costs but the presence of a transfusional centre is mandatory. The procedure starts by collecting a whole venous blood sample (40–50 ml) from a patient usually from the cubital vein, and it is mixed with citrate to prevent early clotting. Then, it is centrifuged by the transfusion centre for about 15 min (depending on the centrifugation method), and a small sample is taken in order to determine the absence of contamination. The centrifugation separates blood components such as red cells (which are discarded) and gather and concentrate the platelets and, depending on the type of preparation, the leukocytes. In the end, usually 4–10 ml of PRP solution is gained.
Another crucial point to be taken into consideration is PRP activation, which is the mechanism through which the secretion of granules is induced. One possibility is the simultaneous injection of PRP and 1–2 ml of calcium gluconate solution by a two-way syringe in order to cleave fibrinogen with subsequent polymerization of fibrin monomers (10–40 s after injection). Alternatively, physiological activation can be obtained by injecting the unactivated PRP into the site of fibre injury: the contact with collagen and other tissue factors will activate PRP. Some studies determined that most of the GFs are released within 10 min of activation.58 However, unactivated PRP must also be injected as soon as possible after centrifugation in order to prevent gelification.
In our experience, exogenously activated PRP may be used in particular in small, focal grade II intramuscular strain, where its potential role in the promotion of the fibrin clot may help to both accelerate the cellular phase and favour the regenerative process; in such cases, 2–6 ml of PRP could be enough even if the amount of injected solution must be related to the dimension of the injury. In case of larger lesions and/or in particular involving the epymisium and/or the fascial planes, up to 10 ml of PRP may be injected seeking endogenous physiological activation through its contact with collagen and other inflammatory mediators into the site of the injury.
General workflow for ultrasound-guided platelet-rich plasma injection
Pre-interventional planning including an organized tray with all needed materials and preliminary ultrasound evaluation of the lesion.
Proper patient positioning and sterility procedures as already described.
Venous blood sample collection and coagulation prevention.
Centrifugation of the blood sample in order to obtain platelet-rich concentrates/L-PRP solutions.
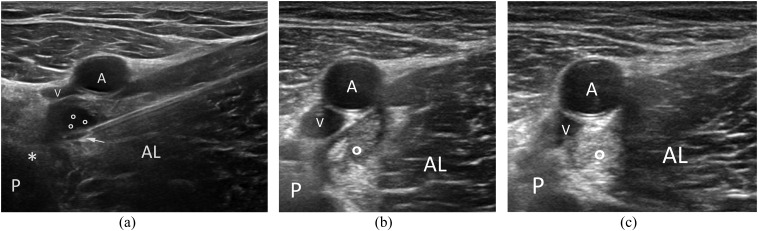
Needle insertion into the site of injury under ultrasound guidance (Figure 4a).
Autologous L-PRP/commercially available L-PRP injection through a two-way syringe: if pre-activation is desired, calcium gluconate and L-PRP solution should be injected simultaneously under ultrasound guidance.
Needle extraction, plaster positioning and ice application for few minutes.
Figure 4.
Platelet-rich plasma injection in a myotendinous tear of adductors. (a) Needle insertion a few millimetres under the vascular bundle; (b) Follow-up at 10 days showing a clot at the site of the treatment (circle); (c) follow-up at 20 days showing an almost homogeneous regenerative tissue at the site of the tear (circle). AL, adductor longus; P, pubis; *, common adductors tendon; A,V, vascular bundle; circles, haematoma.
The timing of injections is another debated point among authors; the proposed protocols are variable in terms of number of injections and interval between each ones: in this setting, one to three injections performed weekly after muscular injury, in relation to the site and entity of the muscular lesion, could be optimal in clinical practice (Figure 4b,c).
The role of PRP in the treatment of MSK pathologies was investigated in many studies;42,58–67 in vitro evidence supports the clinical applications and gives clear indications to the potential mechanisms of PRP.16,49,68–70 Some clinical studies focused on tendinopathy and demonstrated the validity of such a therapeutic option.71–74 However, other studies on large series have reported that PRP treatment of tendinopathy is no more effective than placebo.75–77
This consideration could be partially translated to also muscular injuries: the effects of PRP formulations to favour muscle healing were reported in many case series showing variable results.78–80 In particular, two recent randomized controlled trials reported divergent results.81,82 Published data indicate that, to date, the clinical use of PRP preparations remain widely debated in the literature, probably because of the large quantity of different protocols and preparations to be used in relatively variable clinical settings with different rehabilitative management. These issues need to be addressed before PRP can be used routinely, and further research is warranted.
New perspectives—stem cell injection
Although PRP itself represents an interesting challenge for the next years, stem cell (SC) applications could also play a promising role in sports injuries. They are undifferentiated cells with the intrinsic ability to differentiate into different cell types.37 The rationale behind its use resides in the physiological healing process; PRP releases mediators with the aim of modulating the inflammatory response to injury and promoting cellular regeneration. On the other hand SC can be directly injected to speed up the regeneration process. Hence, the applications are similar to those for PRP alone with the added benefit of regenerative cell enrichment.37,83 To date, considering ethical concerns and non-reported cancerogenic potential, mesenchymal stem cells (MSCs) represent the most promising SC type to be used;37,84 they are multipotent cells which can be obtained by different tissues, such as the adipose tissue, synovial tissue and bone marrow,85 and could be used as cell therapy by cell suspension injections alone or in combination with PRP preparations. Results from in vitro and animal studies indicate the potential use of MSCs to improve tendon and muscle regeneration,83,86–90 but, in our knowledge, only some pilot clinical trials have been conducted,90–92 indicating the need of further studies to reveal the actual effectiveness of MSC therapy.
CONCLUSION
In summary, percutaneous treatment of sport-related muscle injuries has been demonstrated to be effective in treating a number of pathologic conditions. The use of ultrasound guidance further improves the effectiveness of such treatments, especially when dealing with PRP and aspiration of large and partially coagulated haematomas.
Contributor Information
Davide Orlandi, Email: davideor@hotmail.it.
Angelo Corazza, Email: angelcoraz@libero.it.
Alice Arcidiacono, Email: alice.arcidiacono@hotmail.com.
Carmelo Messina, Email: carmelomex@gmail.com.
Giovanni Serafini, Email: giovanni.serafini52@gmail.com.
Luca M Sconfienza, Email: io@lucasconfienza.it.
Enzo Silvestri, Email: silvi.enzo@gmail.com.
REFERENCES
- 1.Micu MC, Vlad VM, Bolboacă SD, Cârlig M, Bodizs GI, Duţu AG, et al. Musculoskeletal ultrasound guided manoeuvres—a security profile. Med Ultrason 2014; 16: 214–21. [DOI] [PubMed] [Google Scholar]
- 2.Orlandi D, Corazza A, Silvestri E, Serafini G, Savarino EV, Garlaschi G, et al. Ultrasound-guided procedures around the wrist and hand: how to do. Eur J Radiol 2014; 83: 1231–8. doi: 10.1016/j.ejrad.2014.03.029 [DOI] [PubMed] [Google Scholar]
- 3.Orlandi D, Corazza A, Fabbro E, Ferrero G, Sabino G, Serafini G, et al. Ultrasound-guided percutaneous injection to treat de Quervain's disease using three different techniques: a randomized controlled trial. Eur Radiol 2015; 25: 1512–19. doi: 10.1007/s00330-014-3515-0 [DOI] [PubMed] [Google Scholar]
- 4.Orlandi D, Fabbro E, Mauri G, Savarino E, Serafini G, Sconfienza LM. RE: a simple technique to restore needle patency during percutaneous lavage and aspiration of calcific rotator cuff tendinopathy. PM R 2013; 5: 633. doi: 10.1016/j.pmrj.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 5.Sconfienza LM, Serafini G, Silvestri E. eds. Ultrasound-guided musculoskeletal procedures-the upper limb. Milan, Italy: Springer-Verlag; 2012. pp. 1–9. [Google Scholar]
- 6.Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle us-guided percutaneous treatment–nonrandomized controlled trial. Radiology 2009; 252: 157–64. doi: 10.1148/radiol.2521081816 [DOI] [PubMed] [Google Scholar]
- 7.Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop 2011; 2: 57–66. doi: 10.5312/wjo.v2.i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naredo E, Cabero F, Beneyto P, Cruz A, Mondéjar B, Uson J, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol 2004; 31: 308–14. [PubMed] [Google Scholar]
- 9.Sibbitt WL, Jr, Peisajovich A, Michael AA, Park KS, Sibbitt RR, Band PA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol 2009; 36: 1892–902. doi: 10.3899/jrheum.090013 [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Oliva F, Frizziero A, Nanni G, Barazzuol M, Via AG, et al. ISMuLT guidelines for muscle injuries. Muscles Ligaments Tendons J 2014; 3: 241–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Corazza A, Orlandi D, Baldari A, Gatto P, Stellatelli M, Mazzola C, et al. Thigh muscles injuries in professional soccer players: a one year longitudinal study. Muscles Ligaments Tendons J 2014; 3: 331–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Koulouris G, Connell D. Hamstring muscle complex: an imaging review. Radiographics 2005; 25: 571–86. doi: 10.1148/rg.253045711 [DOI] [PubMed] [Google Scholar]
- 13.Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med 2010; 3: 26–31. doi: 10.1007/s12178-010-9064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstrand J, Healy JC, Waldén M, Lee JC, English B, Hägglund M. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 2012; 46: 112–17. doi: 10.1136/bjsports-2011-090155 [DOI] [PubMed] [Google Scholar]
- 15.Delos D, Maak TG, Rodeo SA. Muscle injuries in athletes: enhancing recovery through scientific understanding and novel therapies. Sports Health 2013; 5: 346–52. doi: 10.1177/1941738113480934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J 2012; 32: 150–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Szopinski KT, Smigielski R. Safety of sonographically guided aspiration of intramuscular, bursal, articular and subcutaneous hematomas. Eur J Radiol 2012; 81: 1581–3. doi: 10.1016/j.ejrad.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 18.Draghi F, Robotti G, Jacob D, Bianchi S. Interventional musculoskeletal ultrasonography: precautions and contraindications. J Ultrasound 2010; 13: 126–33. doi: 10.1016/j.jus.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peetrons P. Ultrasound of muscles. Eur Radiol 2002; 12: 35–43. doi: 10.1007/s00330-001-1164-6 [DOI] [PubMed] [Google Scholar]
- 20.Vanhoenacker FM, Maas M, Jielen JL. eds. Imaging of orthopedic sports injuries. Berlin, Germany: Springer-Verlag; 2007. [Google Scholar]
- 21.McNally E. ed. Practical musculoskeletal ultrasound. London, UK: Churchill Livingston; 2004. [Google Scholar]
- 22.Alessandrino F, Balconi G. Complications of muscle injuries. J Ultrasound 2013; 16: 215–22. doi: 10.1007/s40477-013-0010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi S, Martinoli C, Abdelwahab IF, Derchi LE, Damiani S. Sonographic evaluation of tears of the gastrocnemius medial head (“tennis leg”). J Ultrasound Med 1998; 17: 157–62. [DOI] [PubMed] [Google Scholar]
- 24.Kwak HS, Han YM, Lee SY, Kim KN, Chung GH. Diagnosis and follow-up US evaluation of ruptures of the medial head of the gastrocnemius (“tennis leg”). Korean J Radiol 2006; 7: 193–98. doi: 10.3348/kjr.2006.7.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson P. Essential radiology for sports medicine. London, UK: Springer; 2010. [Google Scholar]
- 26.Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine 2012; 79: 192–94. doi: 10.1016/j.jbspin.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Hansford BG, Stacy GS. Musculoskeletal aspiration procedures. Semin Intervent Radiol 2012; 29: 270–85. doi: 10.1055/s-0032-1330061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morvan G, Vuillemin V, Guerini H. Interventional musculoskeletal ultrasonograpjy of the lower limb. Diagn Interv Imaging 2012; 93: 652–64. doi: 10.1016/j.diii.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 29.Hamada M, Shimizu Y, Aramaki-Hattori N, Kato T, Takada K, Aoki M, et al. Management of chronic expanding haematoma using triamcinolone after latissimus dorsi flap harvesting. Arch Plast Surg 2015; 42: 218–22. doi: 10.5999/aps.2015.42.2.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi S, Martinoli C, eds. Ultrasound of the musculoskeletal system. Berlin, Germany: Springer-Verlag; 2007. [Google Scholar]
- 31.Taïeb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas. Eur J Radiol 2009; 72: 44–9. doi: 10.1016/j.ejrad.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 32.Bermejo A, Diaz De Bustamante T, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics 2013; 33: 833–55. doi: 10.1148/rg.333115062 [DOI] [PubMed] [Google Scholar]
- 33.Bonilla-Yoon I, Masih S, Patel DB, White EA, Levine BD, Chow K, et al. The Morel-Lavallée lesion: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol 2014; 21: 35–43. doi: 10.1007/s10140-013-1151-7 [DOI] [PubMed] [Google Scholar]
- 34.Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallée lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med 2007; 35: 1162–7. doi: 10.1177/0363546507299448 [DOI] [PubMed] [Google Scholar]
- 35.Dawre S, Lamba S, Harinath S, Gupta S, Gupta A. The Morel-Lavallée lesion: a review and a proposed algorithmic approach. Eur J Plast Surg 2012; 35: 489–94. doi: 10.1007/s00238-012-0725-z [DOI] [Google Scholar]
- 36.Nickerson TP, Zielinski MD, Jenkins DH, Schiller HJ. The Mayo clinic experience with Morel-Lavallée lesions: establishment of a practice management guideline. J Trauma Acute Care Surg 2014; 76: 493–7. doi: 10.1097/TA.0000000000000111 [DOI] [PubMed] [Google Scholar]
- 37.Guevara-Alvarez A, Schmitt A, Russell RP, Imhoff AB, Buchmann S. Growth factor delivery vehicles for tendon injuries: mesenchymal stem cells and platelet rich plasma. Muscles Ligaments Tendons J 2014; 4: 378–85. [PMC free article] [PubMed] [Google Scholar]
- 38.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004; 62: 489–96. doi: 10.1016/j.joms.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 39.Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet 1990; 170: 56–60. [PubMed] [Google Scholar]
- 40.Rizzo C, Vetro R, Vetro A, Mantia R, Iovane A, Di Gesù M, et al. The role of platelet gel in osteoarticular injuries of young and old patients. Immun Ageing 2014; 11: 21. doi: 10.1186/s12979-014-0021-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andia I, Rubio-Azpeitia E, Martin JI, Abate M. Current concepts and translational uses of platelet rich plasma biotechnology. In: Ekinci D, ed. Biotechnology: Intech; 2015. pp. 1–31. [Google Scholar]
- 42.Ahmad Z, Howard D, Brooks RA, Wardale J, Henson FM, Getgood A, et al. The role of platelet rich plasma in musculoskeletal science. JRSM Short Rep 2012; 3: 40. doi: 10.1258/shorts.2011.011148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sánchez M, Anitua E, Delgado D, Sánchez P, Orive G, Padilla S. Muscle repair: platelet-rich plasma derivates as a bridge from spontaneity to intervention. Injury 2014; 45(Suppl. 4): S7–14. doi: 10.1016/S0020-1383(14)70004-X [DOI] [PubMed] [Google Scholar]
- 44.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004; 84: 209–38. doi: 10.1152/physrev.00019.2003 [DOI] [PubMed] [Google Scholar]
- 45.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011; 138: 3625–37. doi: 10.1242/dev.064162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shortreed K, Johnston A, Hawke T. Satellite cells and muscle repair. Skelet Muscle Damag Repair 2008; 1: 77–88. [Google Scholar]
- 47.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A 2010; 107: 3287–92. doi: 10.1073/pnas.0903875106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conboy I, Freimer J, Weisenstein L. Tissue engineering of muscle tissue. In: Ducheyne P, ed. Comprehensive biomaterials. Oxford, UK: Elsevier; 2011. pp. 345–59. [Google Scholar]
- 49.Mazzocca AD, McCarthy MB, Chowaniec DM, Dugdale EM, Hansen D, Cote MP, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med 2012; 40: 1742–9. doi: 10.1177/0363546512452713 [DOI] [PubMed] [Google Scholar]
- 50.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 2011; 39: 266–71. doi: 10.1177/0363546510387517 [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann R, Jakubietz R, Jakubietz M, Strasser E, Schlegel A, Wiltfang J, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 2001; 41: 1217–24. doi: 10.1046/j.1537-2995.2001.41101217.x [DOI] [PubMed] [Google Scholar]
- 52.Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med 2011; 39: 729–34. doi: 10.1177/0363546511401576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009; 27: 158–67. doi: 10.1016/j.tibtech.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 54.Dohan Ehrenfest DM, Bielecki T, Mishra A, Borzini P, Inchingolo F, Sammartino G, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol 2012; 13: 1131–7. [DOI] [PubMed] [Google Scholar]
- 55.Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 2014; 4: 3–9. [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol 2012; 13: 1185–95. doi: 10.2174/138920112800624283 [DOI] [PubMed] [Google Scholar]
- 57.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy 2012; 28: 998–1009. doi: 10.1016/j.arthro.2012.04.148 [DOI] [PubMed] [Google Scholar]
- 58.Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R 2011; 3: 226–50. doi: 10.1016/j.pmrj.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 59.Peerbooms JC, van Laar W, Faber F, Schuller HM, van der Hoeven H, Gosens T. Use of platelet rich plasma to treat plantar fasciitis: design of a multi centre randomized controlled trial. BMC Musculoskelet Disord 2010; 11: 69. doi: 10.1186/1471-2474-11-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson JJ, Lee KS, Miller AT, Wang S. Platelet-rich plasma for the treatment of chronic plantar fasciopathy in adults: a case series. Foot Ankle Spec 2014; 7: 61–7. doi: 10.1177/1938640013509671 [DOI] [PubMed] [Google Scholar]
- 61.Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double- blind randomized controlled trial: platelet-rich plasma versus cortico- steroid injection with a 1-year follow-up. Am J Sports Med 2010; 38: 255–62. doi: 10.1177/0363546509355445 [DOI] [PubMed] [Google Scholar]
- 62.Andia I, Maffulli N. Muscle and tendon injuries: the role of biological interventions to promote and assist healing and recovery. Arthroscopy 2015; 31: 999–1015. doi: 10.1016/j.arthro.2014.11.024 [DOI] [PubMed] [Google Scholar]
- 63.Kon E, Filardo G, Delcogliano M, Presti ML, Russo A, Bondi A, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury 2009; 40: 598–603. doi: 10.1016/j.injury.2008.11.026 [DOI] [PubMed] [Google Scholar]
- 64.Hrnack SA, Barber FA, Hapa O. Rotator cuff repairs augmented by platelet rich plasma evaluated with magnetic resonance imaging and clinical outcomes (SS-08). Arthroscopy 2010; 26: e4–5. doi: 10.1016/j.arthro.2010.04.018 [DOI] [Google Scholar]
- 65.Sanchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol 2008; 26: 910–13. [PubMed] [Google Scholar]
- 66.Sariguney Y, Yavuzer R, Elmas C, Yenicesu I, Bolay H, Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg 2008; 24: 159–67. doi: 10.1055/s-2008-1076752 [DOI] [PubMed] [Google Scholar]
- 67.Creaney L, Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med 2008; 42: 314–20. doi: 10.1136/bjsm.2007.040071 [DOI] [PubMed] [Google Scholar]
- 68.Beitzel K, McCarthy MB, Russell RP, Apostolakos J, Cote MP, Mazzocca AD. Learning about PRP using cell-based models. Muscles Ligaments Tendons J 2014; 4: 38–45. [PMC free article] [PubMed] [Google Scholar]
- 69.Anitua E, Andia I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res 2005; 23: 281–6. [DOI] [PubMed] [Google Scholar]
- 70.de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 2008; 36: 1171–8. doi: 10.1177/0363546508314430 [DOI] [PubMed] [Google Scholar]
- 71.Del Buono A, Papalia R, Denaro V, Maccauro G, Maffulli N. Platelet rich plasma and tendinopathy: state of the art. Int J Immunopathol Pharmacol 2011; 24(1 Suppl. 2): 79–83. [DOI] [PubMed] [Google Scholar]
- 72.Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop 2010; 34: 909–15. doi: 10.1007/s00264-009-0845-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaweda K, Tarczynska M, Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med 2010; 31: 577–83. doi: 10.1055/s-0030-1255028 [DOI] [PubMed] [Google Scholar]
- 74.Ferrero G, Fabbro E, Orlandi D, Martini C, Lacelli F, Serafini G, et al. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J Ultrasound 2012; 15: 260–6. doi: 10.1016/j.jus.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma- Zeinstra SM, Verhaar JA, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med 2011; 39: 1623–9. doi: 10.1177/0363546511404877 [DOI] [PubMed] [Google Scholar]
- 76.de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med 2011; 45: 387–92. doi: 10.1136/bjsm.2010.076398 [DOI] [PubMed] [Google Scholar]
- 77.de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 2010; 303: 144–9. doi: 10.1001/jama.2009.1986 [DOI] [PubMed] [Google Scholar]
- 78.Borrione P, Ruiz MTP, Giannini S, Gianfrancesco AD, Pigozzi F. Effect of platelet-released growth factors on muscle strains: a case control report. Med Sport 2011; 64: 317–22. [Google Scholar]
- 79.Hamilton B, Knez W, Eirale C, Chalabi H. Platelet enriched plasma for acute muscle injury. Acta Orthop Belg 2010; 76: 443–8. [PubMed] [Google Scholar]
- 80.Loo WL, Lee DY, Soon MY. Plasma rich in growth factors to treat adductor longus tear. Ann Acad Med Singapore 2009; 38: 733–4. [PubMed] [Google Scholar]
- 81.A Hamid MS, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med 2014; 42: 2410–18. doi: 10.1177/0363546514541540 [DOI] [PubMed] [Google Scholar]
- 82.Reurink G, Goudswaard GT, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, et al. Platelet rich plasma injections in acute muscle injury. N Engl J Med 2014; 370: 2546–7. doi: 10.1056/NEJMc1402340 [DOI] [PubMed] [Google Scholar]
- 83.Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, et al. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med 2011; 39: 1912–22. doi: 10.1177/0363546511415239 [DOI] [PubMed] [Google Scholar]
- 84.Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Appli cation of stem cells in orthopedics. Stem Cells Int 2012; 2012: 394962. doi: 10.1155/2012/394962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCullagh KJ, Perlingeiro RC. Coaxing stem cells for skeletal muscle repair. Adv Drug Deliv Rev 2015; 84: 198–207. doi: 10.1016/j.addr.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One 2011; 6: e17531. doi: 10.1371/journal.pone.0017531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang TF, Yew TL, Chiang ER, Ma HL, Hsu CY, Hsu SH, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med 2013; 41: 1117–25. doi: 10.1177/0363546513480786 [DOI] [PubMed] [Google Scholar]
- 88.Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H. Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am 2010; 92: 2776–84. doi: 10.2106/JBJS.I.01325 [DOI] [PubMed] [Google Scholar]
- 89.Rinaldi F, Perlingeiro RC. Stem cells for skeletal muscle regeneration: therapeutic potential and roadblocks. Transl Res 2014; 163: 409–17. doi: 10.1016/j.trsl.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cezar CA, Mooney DJ. Biomaterial-based delivery for skeletal muscle repair. Adv Drug Deliv Rev 2015; 84: 188–97. doi: 10.1016/j.addr.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Connell D, Datir A, Alyas F, Curtis M. Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. Br J Sports Med 2009; 43: 293–8. doi: 10.1136/bjsm.2008.056457 [DOI] [PubMed] [Google Scholar]
- 92.Clarke AW, Alyas F, Morris T, Robertson CJ, Bell J, Connell DA. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am J Sports Med 2011; 39: 614–23. doi: 10.1177/0363546510387095 [DOI] [PubMed] [Google Scholar]