Abstract
Mycotic keratitis is an ocular infective process derived from any fungal species capable of corneal invasion. Despite its rarity in developed countries, its challenging and elusive diagnosis may result in keratoplasty or enucleation following failed medical management. Filamentous fungi such as Fusarium are often implicated in mycotic keratitis. Bearing greater morbidity than its bacterial counterpart, mycotic keratitis requires early clinical suspicion and initiation of antifungal therapy to prevent devastating consequences. We describe a case of multidrug-resistant mycotic keratitis in a 46-year-old man who continued to decline despite maximal therapy and therapeutic keratoplasty. Finally, enucleation was performed as a means of source control preventing dissemination of a likely untreatable fungal infection into the orbit. Multidrug-resistant Fusarium is rare, and may progress to endophthalmitis. We discuss potential management options which may enhance diagnosis and outcome in this condition.
Background
Keratitis is an inflammation of the cornea, caused by invasion of the corneal epithelium, stroma and, in more severe disease, endothelium and anterior chamber of the eye. It is caused by a myriad of organisms, notably bacterial, but can also include viral, fungal and amoebic agents.1 Corneal infections remain an important cause of ocular morbidity and blindness worldwide.2 3 Despite its lower incidence, fungal keratitis (mycotic keratitis) represents an elusive and diagnostic challenge to identify and treat, compared with its bacterial counterparts, resulting in a higher rate of comorbidity. This is in large part due to its insidious onset, diversity of clinical presentation, deep corneal penetrating capabilities resulting in weakened eradication response to medical therapy and protracted laboratory incubation periods.4 The progressive tissue damage/inflammatory reaction resulting from delayed presentation, diagnosis and treatment may result in irreversible vision loss due to corneal scarring and potential loss of the eye from corneal perforation or intraocular spread of the infection requiring surgical interventions such as therapeutic penetrating keratoplasty (PK) or enucleation. The causative pathogens associated with mycotic keratitis vary worldwide, being more common in developing nations (China and India) and tropical and agricultural regions, accounting for almost half of all microbial keratitis in these regions.4–6 Recent studies have suggested an increasing prevalence of disease.7 In contrast, it is relatively uncommon in developed nations (Australia and the UK), accounting for only 1–5% of all infectious keratitis presentations.5 Mycotic keratitis is most frequently associated with either filamentous (Fusarium spp, Aspergillus spp, Penicillium spp and Curvularia spp) or yeast-like fungi (Candida albicans and other Candida species).8 9 Furthermore, there appears to be a strong geographical predilection between subgroups, with filamentous fungi predominating in tropical latitudes, while Candida species prevail in temperate climates.9 Several predisposing factors have been associated with mycotic keratitis including lower socioeconomic status, disruption of innate corneal immunity (ocular trauma, particularly vegetative or soil-contaminated objects, corneal surgery or chronic ocular surface disease), contact lens wear and immunosuppressive conditions or treatment (diabetes mellitus, HIV and previous corticosteroid topical use).1 9 Mycotic keratitis usually presents in an insidious manner and may mimic a bacterial aetiology.10 Filamentous fungi are typified by grey–white ‘satellite’ stromal infiltrates with irregular feathery margins and raised sloughy borders, hyphate lines extending beyond ulcer edges into normal cornea, along with suppuration, hypopyon, Descemet's fold and an immune ring with minimal surrounding infiltrate and minimal cellular infiltration in adjacent stroma.1 9 Despite systematic reviews including randomised control trials, the gold standard for the treatment of fungal keratitis remains elusive.4 7 11 Current treatment modalities rely on empiric, broad spectrum medical (topical, intraocular or systemic therapy, commonly amphotericin B, natamycin and voriconazole) or surgical means (periodic necrotic tissue debridement; conjunctival flap; lamellar or PK or enucleation following primary medical failure).9 Corneal collagen crosslinking (CXL) has been reported as an emerging treatment for infectious keratitis, although with variable outcomes in the setting of fungal keratitis.12 13 Compounding this issue is the emergence of resistant species; prolonged (days to weeks) microbiological identification of causative/offending organism; cost and limited availability in organising susceptibility testing and difficulty in their interpretation; protracted response (period of weeks) to medical therapy; individual therapeutic efficacy, toxicity and penetrance (problematic in deeper set lesions); ultimate eradication reliant on host defence mechanism.8 9 14 We report a case of multidrug-resistant Fusarium solani keratitis progressing to endophthalmitis despite maximal medical therapy and requiring enucleation as a means of source control.
Case presentation
A 46-year-old otherwise healthy man presented to emergency department department at the Gold Coast University Hospital (GCUH, Queensland, Australia) with atraumatic acute-onset severe right eye pain, photophobia and blurred vision. Other than previous blunt trauma (eye vs marble) to his right eye in his younger years, he was otherwise healthy and did not have obvious risk factors for keratitis such as contact lens use, trauma, or immunosuppression conditions or treatment. Clinical examination revealed a best-corrected visual acuity of 6/12 right eye (OD). A large stellate-shaped epithelial defect (4×3 mm) overlying the inferior central visual axis (figure 1A) with grey/white subepithelial feathery infiltrates (3×0.5 mm) in a ring formation was noted. Conjunctival injection, dilated iris vessels and a mild anterior chamber reaction (cells 1+, flare 1+) with a small hypopyon (0.3 mm) were observed. His other eye was normal with a visual acuity of 6/6 left eye (OS). Although a working diagnosis of keratitis of unknown origin was made, a bacterial source was suspected and he was started on fortified antimicrobial regime (hourly topical cephalothin (5%) and ofloxacin (0.3%)). The clinical scenario waxed and waned over the course of 1 week with initial and subsequent attempts (by means of corneal scraping sent for stain and culture and serological testing including herpes simplex virus (HSV) and varicella-zoster virus (VZV)) failing to elucidate the offending pathogen. Despite his fortified antimicrobial treatment, his condition worsened in his right eye (ocular pain, perception to light visual acuity, expanding epithelial defect with increasing subepithelial infiltrates, worsening anterior chamber reaction and purulent discharge), raising the possibility of an atypical aetiology including fungal or amoebic agents (figure 1B). Revision of his medical management on days 8–11 of his admission included broadening of antimicrobial cover, initiation of topical polyhexamethylene biguanide, brolene, voriconazole and systemic valacyclovir. Repeated testing, which resulted in a total of three separate corneal scrapes (days 1, 6 and 9) sent for calcofluor white, Grocott and fungal cultures; four corneal tissue biopsies (days 9, 15 (×2) and 26); PCR: viral (days 1 and 5: HSV 1/2, VZV), chlamydia and gonorrhoea (day 1), panfungal (days 10, 16; targeting all fungal ribosomal RNA) and Acanthamoeba (days 10 and 16); and one anterior chamber aqueous tap (day 21), was performed during admission.
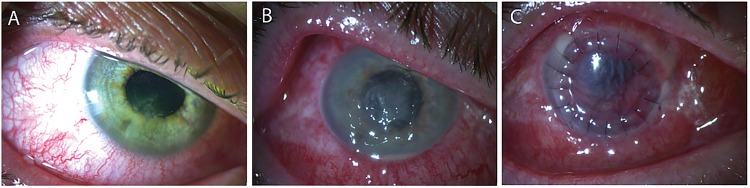
Figure 1.
Slit-lamp photographs. Notes: (A) a large stellate-shaped epithelial defect with multiple stromal satellite infiltrates overlying the inferior visual axis on presentation. The grey–white stromal infiltrates had feathery margins and raised sloughy borders with associated conjunctival injection, dilated iris vessels and a mild anterior chamber reaction. (B) Clinical deterioration: enlarging central epithelial defect and coalescing stromal infiltrates with pronounced conjunctival injection, corneal oedema and hypopyon. Poor treatment response required revision of medical management to include broadening antimicrobial cover and treatment consideration of atypical agents including viral, fungal and Acanthamoeba. (C) Therapeutic penetrating keratoplasty performed on day 26. Fungal invasion seen on the iris intraoperatively.
With repeatedly negative findings, fungal elements were finally identified on day 17 from a corneal biopsy (performed on day 9 of admission), with F. solani cultured on day 26 from an aqueous tap sample. Treatment was tailored accordingly and included topical (1% voriconazole and 5% natamycin), intraocular (intracameral (days 18, 21–25), intrastromal (day 24) and intravitreal voriconazole (200 µg in 0.1 mL; day 26); intracameral (0.01 mg in 0.1 mL), intrastromal (0.05 mg in 0.1 mL) and intravitreal (0.01 mg in 0.1 mL) amphotericin B on day 26), and systemic treatment (oral voriconazole 200 mg twice daily; initiated day 16, ceased day 30 of admission). He also underwent anterior chamber voriconazole irrigation (days 15 and 20; 250 mL of 100 µ/0.1 mL; days 20 and 25). Therapeutic PK was performed on day 26 (figure 1C), with intraoperative findings of a fungating infiltrative white mass stranding seen on an already ischaemic iris. Several operative specimens sent to the National Mycology Reference Centre (SA Pathology, South Australia, Australia) to aid in fungal isolation and susceptibility testing identified a multidrug-resistant F. solani organism on day 26 (minimum inhibitory concentration in microgram per millilitre: amphotericin B, 4; voriconazole, 8; posaconazole, 8; natamycin not tested). A B-scan ultrasound on day 28 identified posterior spread with intravitreal hyperechogenic infiltrates and retina and choroidal thickening.
In the light of several factors including delayed culture diagnosis; poor response to maximal medical therapy (including intracameral); intraoperative findings during PK procedure; identification of a multidrug-resistant organism and B-scan confirmation of a progression to endophthalmitis, a multidisciplinary team approach was sought. This included ophthalmology, microbiology and infectious disease representatives who reached a verdict, recommending enucleation to avoid risk of extrascleral or orbital spread. The patient consented and underwent enucleation on day 29 of admission.
Outcome and follow-up
Following enucleation, the patient recovered well and was discharged home 5 days postprocedure with no further sequelae. He is currently awaiting a right orbital ball implant with reconstructive orbital procedure.
Discussion
Fungal keratitis may carry a poor visual prognosis, and is relatively common in warm climates and developing countries, accounting for almost half of all corneal ulcers in these regions.4–6 Of all causative organisms, fungi remain one of the most elusive and challenging to diagnose and subsequently to treat due to its insidious onset; ability to masquerade; prolonged (days to weeks) microbiological identification of offending organism; the cost associated with, in addition to, the availability of susceptibility testing and difficulty in their interpretation; lack of gold standard therapy; slow response (period of weeks to months) to medical therapy; individual therapeutic efficacy; medical toxicity and penetrance; and ultimate eradication reliant on the host defence mechanism.
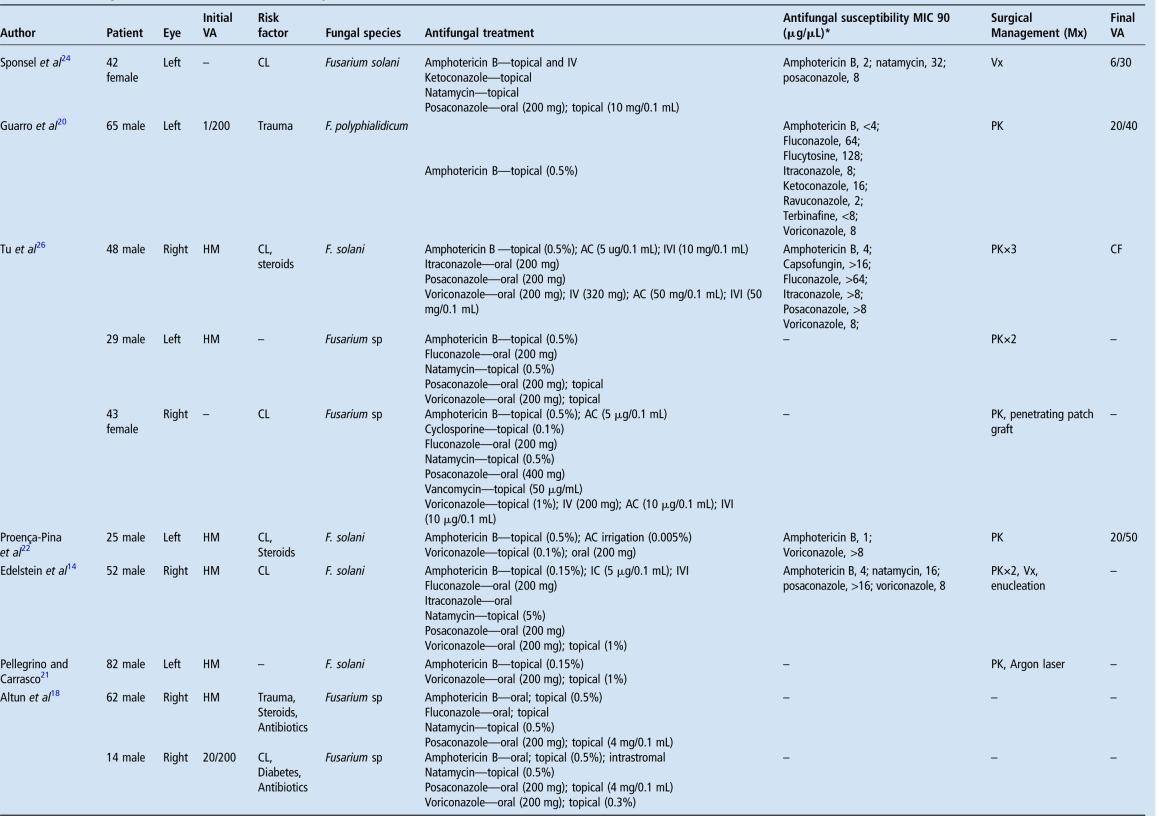
The most commonly implicated mycotic keratitis are filamentous in nature (Fusarium and Aspergillum) with this subgroup having a predilection for tropical climates such as the one found in Queensland, Australia. The described presentation of F. solani keratitis highlighted several issues, including multidrug resistance, which ultimately resulted in a poor clinical outcome and subsequent review of standard procedures at this tertiary hospital. This patient underwent maximal medical treatment, which included broad spectrum topical, intraocular and systemic antifungal therapy. While the recent literature has shown some efficacy in using intrastromal voriconazole in treating deep recalcitrant fungal keratitis, his condition worsened despite multiple intracameral, intrastromal and intravitreal injections.15 Furthermore, he underwent therapeutic keratoplasty before requiring enucleation in an attempt to eradicate infection of a resistant fungal infection. Multidrug-resistant Fusarium keratitis is rare, with a review of the literature highlighting 15 reported cases (table 1).14 16–26 Of these, it was more prevalent among men (66%) with a mean age of 51 years (range 14–82 years). Contact lens usage was the most common risk factor reported (seven cases), followed by topical steroids (four cases) and trauma (three cases). Drug-resistant Fusarium keratitis was associated with poor visual acuity on presentation (hand movements in 6 of the 8 cases reported) and profound morbidity: 10 cases required keratoplasty, 2 of which proceeded to include enucleation or evisceration, with an additional case requiring enucleation as the sole surgical management. Furthermore, in vitro studies have demonstrated F. solani capable of forming biofilms, resulting in increased resistance to tested antifungal agents, natamycin being the most effective.3
Table 1.
Multidrug-resistant Fusarium keratitis summary
 |
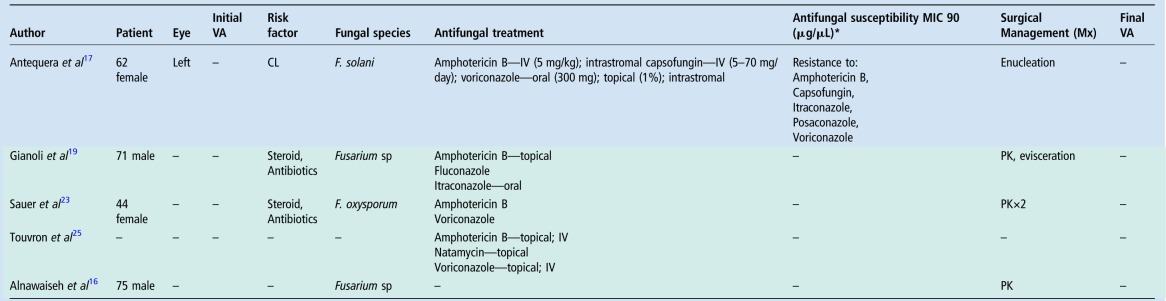 |
Green shaded areas represent articles written in either French or German.
*MIC 90 indicates minimum inhibitory concentration required to inhibit growth of 90% of organisms.
AC, anterior chamber; CF, count fingers; CL, contact lens; HM, hand movement; IC, intracameral; IV, intravenous; IVI, intravitreal; Mx, management; PK, penetrating keratoplasty; VA, visual acuity; Vx, vitrectomy.
Unfortunately, no single treatment for fungal keratitis has emerged as the best and most cost-effective agent, with current management routinely using a combination of systemic, topical and intraocular (intracameral, intrastromal and intravitreal) administration of antifungals, the most common being amphotericin B, voriconazole and natamycin.4 While natamycin is a first-line agent against Fusarium infection, it is less effective against yeast and its efficacy in deeper keratitis is limited owing to its topical administration.4 7
Furthermore, natamycin susceptibility testing is not routinely available in Australia due to cost issues and availability (compound availability and test standardisation; Sarah Kidd, National Mycology Reference Centre Microbiology and Infectious Diseases SA Pathology, personal communication). Amphotericin B displays the highest inhibitory effect against Fusarium, however shows significant variability within this species.27 Alternatively, voriconazole has various routes for administration and has a wider antifungal coverage, active against filamentous and yeast. However, its efficacy as monotherapy in filamentous keratitis is not recommended, being associated with a higher rate of corneal perforation and PK.11 28 Furthermore, while the main treatment modality for mycotic keratitis is topical administration, overall antifungal toxicity and side effects from systemic administration with these agents can be problematic, further adding to disease morbidity.7 11 Finally, corneal collagen CXL (if available) is emerging as an adjunctive therapy to medical and surgical options for the management of microbial keratitis.12 13
Prior to this presentation of fungal keratitis, this tertiary hospital had no formal diagnostic and therapeutic protocol for mycotic keratitis cases. Following the poor outcome from this presentation and subsequent literature review, the GCUH formalised, in consultation with its microbiologist colleagues, a new protocol for the management of suspected fungal keratitis cases (figure 2).29 First, suspect fungal keratitis if risk factors are present (ocular trauma including organic sources, ocular surgery, chronic surface disease, contact lens wear and immunosuppressive environments). Second, if clinical history and examination (serrated infiltrate ulcer margins and raised slough with satellite lesions and anterior chamber reaction) suggest possible fungal involvement, perform early and thorough investigations of the ulcerative material including corneal scrapes (to be placed on two slides, one for gram stain and one for urgent Grocott stain) and microbial swabs (agar gel without charcoal sent) sent for plating for fungal species.10 Additional routine microbial keratitis investigations should concomitantly be performed (bacterial, viral or possibly amoebic-specific studies). Systemic workup should be performed in patients suspected to be immunocompromised.
Figure 2.
Newly devised protocol. Notes: adapted from fungal keratitis management guidelines (Sandwell and West Birmingham Hospitals, UK).29 DM, diabetes mellitus.
Concurrent fortified antibacterial and antifungal therapy should be started in all suspected (unconfirmed) fungal infections until microbiology results are known. First-line monotherapy using commercially available natamycin in early/non-progressive disease should be considered. Subsequent identification of fungal species may facilitate which agent would be best added for dual therapy (amphotericin B for yeast; voriconazole for mold species) for treatment of severe or unresponsive disease. Furthermore, consideration of susceptibility testing (if available) in severe cases not responding to treatment could help target therapy. Given the penetrating capabilities of fungal infections, repeated corneal scrapes may fail to elucidate causative organisms, thereby requiring corneal biopsies to aid in diagnosis. Passing a sterile silk suture (6-0 to 8-0) through deep stromal infiltrate and subsequent inoculation of culture medium is a simpler and less invasive procedure than the alternative corneal biopsy.30–32 In summary, a rare case presentation of a multidrug-resistant F. solani keratitis associated with failed medical management and progressing to endophthalmitis, required enucleation to clear the infection. This presentation led to the modification of current protocols at this tertiary hospital with the view of enhancing diagnosis and disease morbidity.
Learning points.
Mycotic keratitis may be associated with higher ocular morbidity and poorer visual outcome compared with its bacterial counterpart.
Its challenging and elusive diagnosis often requires early clinician suspicion.
Gold standard for treatment remains elusive; requires empiric-based approach; response to therapy may be variable and is usually protracted.
Microbial identification follows prolonged incubation periods.
Emergence of resistant fungal strains has been reported in the literature.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Karsten E, Watson SL, Foster LJ. Diversity of microbial species implicated in keratitis: a review. Open Ophthalmol J 2012;6:110–24. 10.2174/1874364101206010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badiee P. Mycotic keratitis, a state-of-the-art review. Jundishapur J Microbiol 2013;6:e8561 10.5812/jjm.8561 [DOI] [Google Scholar]
- 3.Zhang X, Sun X, Wang Z et al. Keratitis-associated fungi form biofilms with reduced antifungal drug susceptibility. Invest Ophthalmol Vis Sci 2012;53:7774–8. 10.1167/iovs.12-10810 [DOI] [PubMed] [Google Scholar]
- 4.Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep 2013;7:209–18. 10.1007/s12281-013-0150-110.1007/s12281-013-0150-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neoh CF, Daniell M, Chen SC et al. Clinical utility of capsofungin eye drops in fungal keratitis. Int J Antimicrob Agents 2014;44:96–104. 10.1016/j.ijantimicag.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 6.Punia RS, Kundu R, Chander J et al. Spectrum of fungal keratitis: clinicopathologic study of 44 cases. Int J Ophthalmol 2014;7:114–17. 10.3980/j.issn.2222-3959.2014.01.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu S, Zhao GQ, Lin J et al. Natamycin in the treatment of fungal keratitis: a systematic review and meta-analysis. Int J Ophthalmol 2015;8:597–602. 10.3980/j.issn.2222-3959.2015.03.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kredics L, Narendran V, Shobana CS et al. , Indo-Hungarian Fungal Keratitis Working Group. Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses 2015;58:243–60. 10.1111/myc.12306 [DOI] [PubMed] [Google Scholar]
- 9.Thomas P, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbial Infect 2013;19:210–20. 10.1111/1469-0691.12126 [DOI] [PubMed] [Google Scholar]
- 10.Leck A, Burton M. Distinguishing fungal and bacterial keratitis on clinical signs. Commun Eye Health 2015;28:6–7. [PMC free article] [PubMed] [Google Scholar]
- 11.FlorCruz NV, Evans JR. Medical intervention for fungal keratitis. Cochrane Database Syst Rev 2015;(4):CD004241 10.1002/14651858.CD004241.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan E, Snibson GR, Sullivan L. Treatment of infectious keratitis with riboflavin and ultraviolet-A irradiation. J Cataract Refract Surg 2014;40:1919–25. 10.1016/j.jcrs.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Chan TC, Agarwal T, Vajpayee RB et al. Cross-linking for microbial keratitis. Curr Opin Ophthalmol 2016;27:348–52. 10.1097/ICU.0000000000000271 [DOI] [PubMed] [Google Scholar]
- 14.Edelstein SL, Akduman L, Durham BH et al. Resistant Fusarium keratitis progressing to endophthalmitis. Eye Contact Lens 2012;38:331–5. 10.1097/ICL.0b013e318235c5af [DOI] [PubMed] [Google Scholar]
- 15.Kalaiselvi G, Narayana S, Krishnan T et al. Intrastromal voriconazole for deep recalcitrant fungal keratitis: a case series. Br J Ophthalmol 2015;99:195–8. 10.1136/bjophthalmol-2014-305412 [DOI] [PubMed] [Google Scholar]
- 16.Alnawaiseh M, Böhm M, Idelevich E et al. [Successful treatment of Fusarium-associated keratitis with multiresistant pathogen and multimorbid patient]. Ophthalmoge 2014;111:259–61. 10.1007/s00347-013-2874-2 [DOI] [PubMed] [Google Scholar]
- 17.Antequera P, Garcia-Conca V, Martin-Gonzáles C et al. Multidrug resistant Fusarium keratitis. Arch Soc Optalmol 2015;90:382–4. 10.1016/j.oftal.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 18.Altun A, Kurna SA, Sengor T et al. Effectiveness of posaconazole in recalcitrant fungal keratitis resistant to conventional antifungal drugs. Case Rep Ophthalmol Med 2014;2014:701653 10.1155/2014/701653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianoli F, Guex-Crosier Y, Marchetti O et al. [Anterior segment necrosis in multidrug resistant Fusarium keratomycosis: a case study]. J Fr Ophtalmol 2005;28:498–501. 10.1016/S0181-5512(05)81086-1 [DOI] [PubMed] [Google Scholar]
- 20.Guarro J, Rubio C, Gené J et al. Case of keratitis caused by an uncommon Fusarium species. J Clin Microbiol 2003;41:5823–6. 10.1128/JCM.41.12.5823-5826.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrino F, Carrasco MA. Argon laser phototherapy in the treatment of refractory fungal keratitis. Cornea 2013;32:95–7. 10.1097/ICO.0b013e318256140e [DOI] [PubMed] [Google Scholar]
- 22.Proença-Pina J, Ssi Yan Kai I, Bourcier T et al. Fusarium keratitis and endophthalmitis associated with lens contact wear. Int Ophthalmol 2010;30:103–7. 10.1007/s10792-008-9290-7 [DOI] [PubMed] [Google Scholar]
- 23.Sauer A, Abry F, Lhermitte B et al. [Purulent corneal melting secondary to multidrug-resistant Fusarium oxysporum aggravated by topical corticosteroid therapy]. J Fr Ophtalmol 2008;31:534.e1–5. [DOI] [PubMed] [Google Scholar]
- 24.Sponsel WE, Graybill JR, Nevarez HL et al. Ocular and systemic posaconazole (SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br J Ophthalmol 2002;86:829–30. 10.1136/bjo.86.7.829-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touvron G, Denis D, Doat M et al. [Successful treatment of resistant Fusarium solani keratitis with liposomal amphotericin B]. J Fr Ophtalmol 2009;32:721–6. 10.1016/j.jfo.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 26.Tu EY, McCartney DL, Beatty RF. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592). Am L Ophthalmol 2007;143:222–7. 10.1016/j.ajo.2006.10.048 [DOI] [PubMed] [Google Scholar]
- 27.Kawakami H, Inuzuka H, Hori N et al. Inhibitory effects of antimicrobial agents against Fusarium species. Med Mycol 2015;53:603–11. 10.1093/mmy/myv016 [DOI] [PubMed] [Google Scholar]
- 28.Prajna NV, Krishnan T, Mascarenhas J et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol 2013;131:422–9. 10.1001/jamaophthalmol.2013.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandwell and West Birmingham Hospitals, United Kingdom. Fungal keratitis management protocol. http://bmec.swbh.nhs.uk/wp-content/uploads/2013/03/FUNGAL-KERATITIS.pdf (accessed 20 Jan 2016).
- 30.Anutarapongpan O, O'Brien T. Update on management of fungal keratitis. Clin Microbial 2014;3:168 10.4172/2327-5073.1000168 [DOI] [Google Scholar]
- 31.Bethke W. Meeting the challenge of fungal keratitis. Rev Ophthalmol 2013;20:42. [Google Scholar]
- 32.Sridhar M. Diagnosis and management of microbial keratitis. CME Series-11 New Delhi: All India Ophthalmological Society, 2005. [Google Scholar]