Table 2.
Synthesis of substituted benzyl phosphonates in PEG-400a.
| Entry | Benzyl halide | Product | Yieldb (%) |
|---|---|---|---|
| 1 |  |
9842 | |
| 2 |  |
9243 | |
| 3 |  |
8942 | |
| 4 |  |
9142 | |
| 5 |  |
8844 | |
| 6 |  |
9345 | |
| 7 |  |
9846 | |
| 8 |  |
9147 | |
| 9 |  |
9047 | |
| 10 |  |
9347 | |
| 11 |  |
9048 | |
| 12 |  |
9448 | |
| 13 | 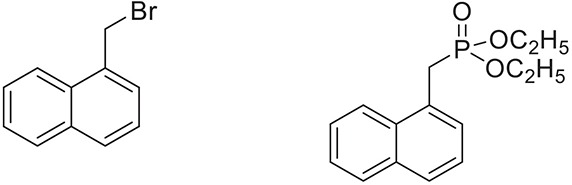 |
8249 | |
Reaction Conditions: benzyl halide (1 mmol) and dialkyl phosphite (1 mmol), K2CO3 (2 mmol), KI (0.3 mmol), PEG (0.5 g), RT (28°C), 6 h.
Isolated Yield.
