Figure 1. Structure of an IgG antibody (Ab).
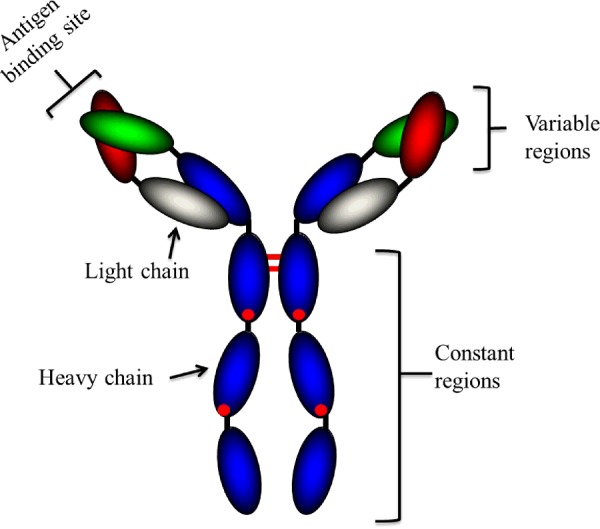
A typical Ab is a large molecule of about 150 kDa made up of four peptide chains. It contains two identical class γ heavy chains of about 50 kDa and two identical light chains of about 25 kDa, and thus a tetrameric quaternary structure. The two heavy chains are linked to each other and to a light chain by disulphide bonds. The resulting tetramer has two identical halves, which together form the Y-like shape. Each end of the fork contains an identical antigen-binding site. (Adapted from [1]).
