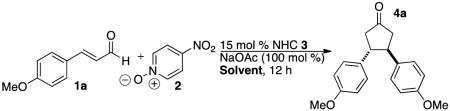
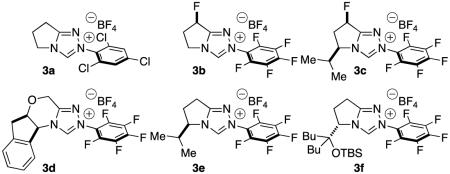
Table 1.
| Entry | NHC | Additive | Temp | Solvent | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|
| 1 | 3a | - | 23 °C | CH2Cl2 | 49 | - |
| 2 | 3b | - | 23 °C | CH2Cl2 | 25 | 12 |
| 3 | 3b | - | 23 °C | PhMe | 28 | 60 |
| 4 | 3c | - | 23 °C | CH2Cl2 | <5 | - |
| 5 | 3d | - | 23 °C | CH2Cl2 | <5 | - |
| 6 | 3e | - | 23 °C | CH2Cl2 | <5 | - |
| 7 | 3b | - | 70 °C | PhMe | 46 | 50 |
| 8 | 3c | - | 70 °C | PhMe | 51 | 84 |
| 9 | 3d | - | 70 °C | PhMe | 30 | 50 |
| 10 | 3e | - | 70 °C | PhMe | 44 | 76 |
| 11 | 3f | - | 70 °C | PhMe | 26 | −78 |
| 12 | 3c | - | 70 °C | PhCF3 | 64 | 84 |
|
| ||||||
| 13 | 3c | LiCl | 70 °C | PhCF3 | 79 | 84 |