Scheme 2.
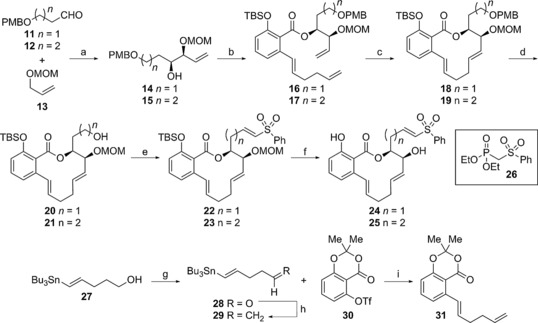
Synthesis of vinyl sulfone analogues 24 and 25: a) 13, sBuLi, THF, −78 °C, then (+)‐β‐methoxydiisopinocamphenylborane, THF, −78 °C to −90 °C, then BF3⋅OEt2, then 11/12, 80 % for 14, 88 % for 15; b) NaHMDS, THF, 0 °C, then 31, THF, 0 °C, then TBSCl, imidazole; c) Grubbs 2nd‐gen. catalyst, PhMe, reflux, 60 %, two steps for 18, 61 %, two steps for 19; d) DDQ, CH2Cl2, 0 °C, 50 % for 20, 63 % for 21; e) SO3⋅Py, Et3N, DMSO, CH2Cl2, 0 °C; f) phosphonate 26, NaH, THF, 0 °C, then aldehyde, 48 %, two steps for 22, 65 %, two steps for 23; f) 4 m HCl, MeOH, 82 % for 24, 85 % for 25; g) SO3⋅Py, Et3N, DMSO, CH2Cl2, 0 °C; h) CH3PPh3Br, tBuOK, 0 °C to RT; i) Pd(PPh3)4, LiCl, THF, 70 °C, 93 %, three steps.
