Figure 2. Rules for writing memory.
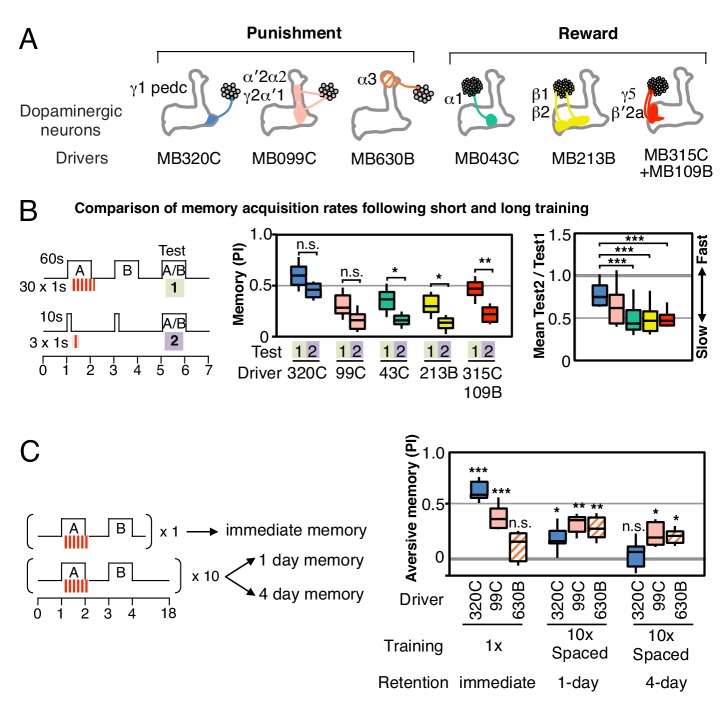
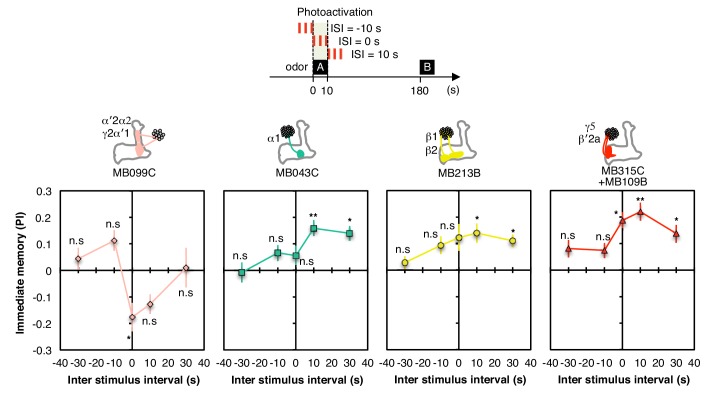
(A) Diagram of the DANs contained in split-GAL4 driver lines, which have been color-coded to facilitate comparison with the plots shown in the subsequent panels. Expression patterns of these drivers, including full confocal stacks, can be found at www.janelia.org/split-gal4. In all experiments, the drivers were crossed with 20xUAS-CsChrimson-mVenus in attP18. (B) Differential effect of training length among DANs. Left: Diagram of the experimental design. Immediate memories formed after paring a 60-s odor presentation with thirty 1-s CsChrimson-activating light pulses (Test 1) were compared with those obtained with a 10-s odor presentation paired with three 1-s light pulses (Test 2). Center: A 60-s training period resulted in significantly better memory performance compared with a 10-s training for MB043C, MB213B and MB315C+MB109B (data from ISI = 0 s for MB099C and ISI =+ 10 s for others drivers were used to provide maximum memory formation; see ISI curves in Figure 2—figure supplement 4). We also observed increased learning with 60-s versus 10-s training (PI of 0.72 versus 0.15) when using R58E02-GAL4 (Liu et al., 2012), a strong GAL4 driver expressed in ~90 PAM cluster DANs that includes all of the ~50 DANs that have expression in MB043C, MB213B and MB315C+MB109B. To facilitate comparison of PI magnitudes, the sign of the PI in this and subsequent panels was reversed for DANs that induced aversive memory (MB320C, MB099C and MB630B). The bottom and top of each box represents the first and third quartile, and the horizontal line dividing the box is the median. The whiskers represent the 10th and 90th percentiles. N = 8–16. Right: Comparison of the effect of training time on memory formation induced by activation of different DANs. Ratios of the mean PI obtained with short training and individual PIs obtained with long training are shown for each driver. Asterisk indicates significance of depicted pairs after comparing all pairs. (C) Comparison of learning after single and repetitive training using the three drivers MB320C, MB099C and MB630B. Either a single training with memory test after 1 min (immediate memory; left) or 10 trainings separated by 15 min resting intervals and then memory tests after 1 (middle) or 4 (right) days were used. Significant aversive 1-day memory was seen with all drivers, while 4-day memory was observed only with MB099C and MB630B. MB320C failed to show 4-day memory despite displaying the most robust immediate memory, while MB630B did not induce significant immediate memory. N = 8–12. Asterisk indicates significance of comparison of indicated pairs in B and from 0 in C: *p<0.05; **p<0.01; ***p<0.001.
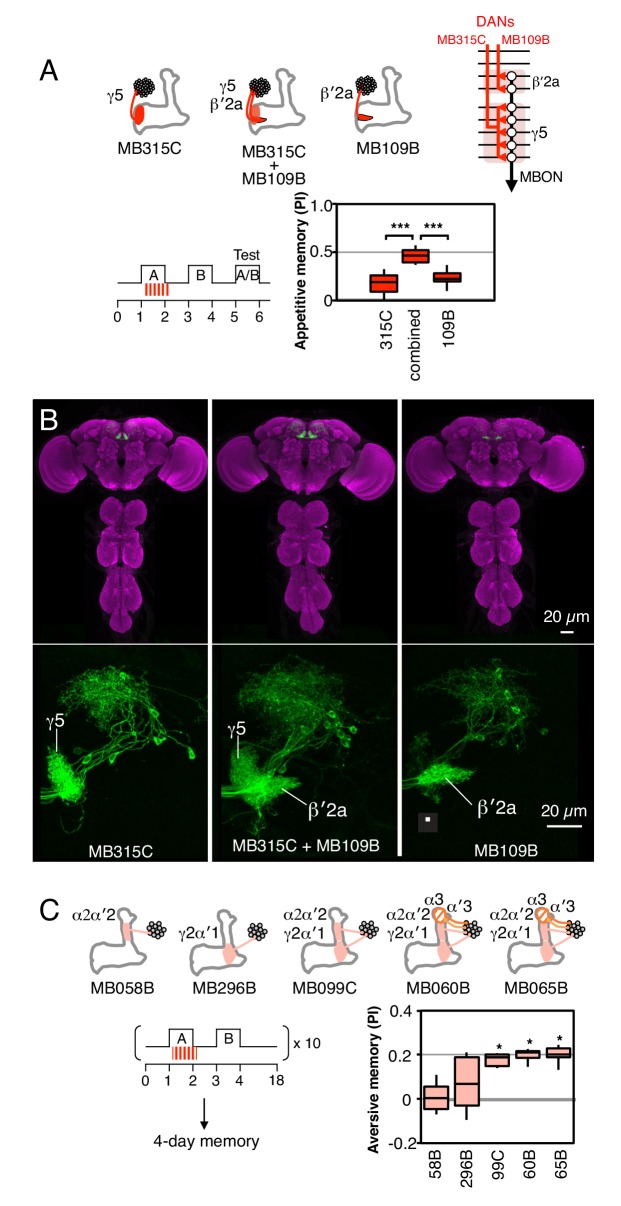
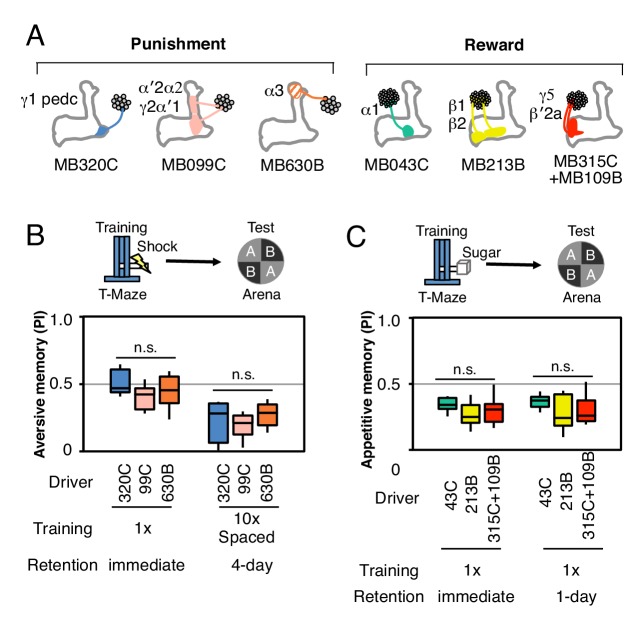
Figure 2—figure supplement 1. Combinatorial roles of DANs in memory formation.
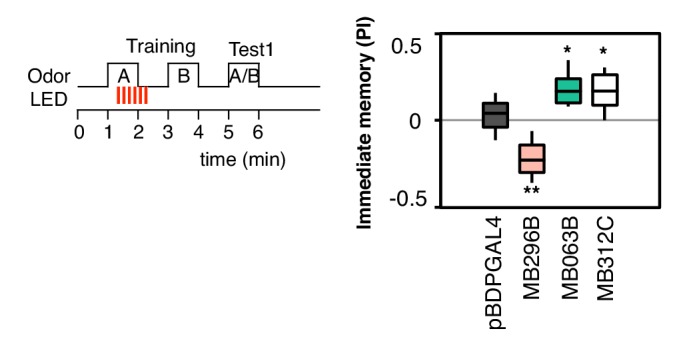
Figure 2—figure supplement 2. Additional drivers that induced weak, but significant, memory.