Abstract
We studied the course of chronic HCV infections in a cohort of 222 persons with hemophilia (PWH) and von Willebrand disease followed at our center since 1973. Twenty two (10%) developed end stage liver disease (ESLD). Forty years after HCV infection, cumulative incidence of ESLD was 12.3% and overall survival was 45.5%. Those who were infected with HCV only (n=100) had a survival of 75.2%, while those infected with HIV (n=122) had a survival of 24% (P<.001). Survivals were significantly longer for those infected with HCV at younger age (< 15 years) compared to those infected over age 30 years (P=0.014). Cause specific deaths for ESLD and bleeding were 8.8% and 8.3% respectively. For HIV negative subjects, the annual hazard of death from ESLD and bleeding was near zero for the first 10 years, and then rose slowly over the next 20 years to 0.4/100py for ESLD and 0.2/100py for bleeding. Sixty subjects completed 79 treatment episodes. Sustained viral response rates increased from 7/21 (33%) between 1990-2001, to 17/29 (58%) between 2002-2011, and to 27/29 (93%), since 2012 with the advent of the directly acting antiviral agents. These results confirm the very slow ESLD progression rate in HIV negative PWH. However, the risk of death from both ESLD and bleeding increases steadily with longer duration of HCV infection. More aggressive surveillance to detect those with early fibrosis is needed now that curative treatment is possible in >95% of individuals.
Keywords: Hemophilia, Hepatitis C, liver disease, survival, treatment
Introduction
Virtually all persons with hemophilia (PWH) who received clotting factor concentrates prior to the implementation of effective viral inactivation techniques by the mid 1980's became infected with hepatitis C virus (HCV) [1-3]. Approximately 80% are estimated to have developed chronic infections, and 20% of these are estimated to progress to cirrhosis. Those with cirrhosis are at risk for hepatic decompensation and continued progression to end stage liver disease (ESLD) [4]. In a report using meta-analysis and meta regression of 111 reported natural history studies, the overall estimated prevalence of cirrhosis 20 years after infection was 16% [5], but estimates varied widely from a high of 34% for post transfusion cohorts and 33% for tertiary-care liver clinics, to a low of 7% for community based cohorts [6,7]. Once cirrhosis is established, hepatocellular carcinoma (HCC) develops at a rate of 1%to 4% per year [8,9]. Twenty-five to 30% of those with chronic HCV have been co-infected with HIV [10,11]. Those who are HIV co-infected have a higher rate of liver disease progression than those who are infected with HCV alone [11,12].
At the Hemophilia Center of Central Pennsylvania, we follow a cohort of PWH who were infected with HCV more than 40 years ago [13]. Herein, we report the long term outcomes and the response to HCV treatment in this group of individuals.
Patients and Methods
The subjects consisted of PWH and von Willebrand disease who have been followed regularly in our comprehensive care clinic since 1973. The study was approved by the institutional review board of the Penn State Hershey Medical Center and all participants gave written informed consent. From the outset, information regarding the first exposure to clotting factor concentrates and other blood products was recorded, and periodic evaluations consisting of an interim history, physical examination, complete blood count, serum alanine aminotransferase (ALT), hepatitis B surface antigen (HBsAg), and hepatitis B antibody (anti HBs) tests were performed at intervals of 6 months to 1 year, as previously described [14]. At each visit, serum and plasma samples were obtained and stored frozen at -20 to -70 degrees C. Tests for HIV antibody were initiated in 1984, for HCV antibody in 1990, for HIV RNA in 1992, and for HCV RNA and HCV genotyping in 1992-1994. Fetal alpha protein, serum bilirubin, alkaline phosphatase, albumin and prothrombin times were performed yearly on HCV RNA positive patients, and abdominal ultrasound examinations and/or CT scans were performed on those at high risk for cirrhosis. End stage liver disease (ESLD) was defined as persistent ascites, bleeding esophageal varices, hepatic encephalopathy, HCC or death excluding non-hepatic causes.
Study enrollment date was the first date that serum was collected with informed consent for HIV or HCV testing. The vast majority of those found to have positive HCV antibody tests were followed prospectively in the first Multicenter Cohort Study (MHCS-1) of participants with HIV infections from 1982 to 1999, and/or the second Multicenter Cohort Study (MCHS -II) of participants with HCV infections from 2000 to 2007.
Dates of first exposure to cryoprecipitate (cryo) or plasma, or to clotting factor concentrates given before December 31, 1987, were used to estimate duration of HCV infection. Dates were self-reported at the time of study enrollment, and recorded at yearly visits on those who were first exposed after enrollment. Those who received non-heated concentrates before 1987 (n=177) were assumed to have been infected at the date of their first exposure. If the first concentrate exposure was after 1987, those who received cryo or fresh frozen plasma (FFP) before that date (n=10) were estimated to have been infected at the midpoint between date of first exposure and 1987. Those who received cryo or FFP but no non-heat treated concentrate (n=35) were estimated to have been infected at the mid-point between that date and December 31, 1987.
Imputed dates of HCV infection using two statistical methods termed EMS2D (expectation maximization algorithm with smoothing in two dimensions) and method B used to estimate individual infection dates were available for comparison on 65 subjects who participated in the MHCS-II study between 2001 and 2005 [15].
Statistical methods
Descriptive statistics were calculated to characterize the study cohort. Dates of first exposure to cryo or plasma, or to clotting factor concentrates given before December 31, 1987, were used to estimate the HCV infection date as described above. Survival rates after HCV infection were estimated by Kaplan-Meier curves, and were compared between groups of interest (e.g., HIV status, age groups) by log-rank tests. Survival time was censored at the last clinical visit date or last known follow-up date for patients who dropped out of study, and at 5/15/2015 for those still active in the study. Cause-specific survival analyses were performed using competing risk methods. Cumulative incidence functions and kernel-smoothed cause-specific hazard functions were estimated for deaths from HIV, liver failure, bleeding and other causes, respectively. Cumulative incidence function measures the overall probability of death from a specific cause in the presence of other competing causes. Cause-specific hazard function represents the instantaneous rate of death due to a specific cause for those who are still at risk. For simple interpretation, hazard rate at a particular time point is described as the number of cases per 100 person years (i.e., the probability of the event at a given year among persons who are free of that event at the beginning of that year). Competing risk methods were also used to estimate the cumulative incidence of developing ELSD with the non-hepatic death treated as competing risk event. Among patients who were on treatments, sustained viral response rates were calculated as the number of successes over the number of treatment episodes across three time periods (1990-2001, 2002-2011, after 2011).
Results
246 subjects were identified as having been infected with HCV by HCV antibody testing at the time of their yearly comprehensive care visits. Eight were excluded for IV drug use. On initial testing, 16 (6.5%) were HCV RNA negative in the absence of treatment, and were assumed to have spontaneously cleared their virus. In 12/16 with retrospective samples available for testing, HCV RNA had cleared in all but one within 1-2 years after the initial infection [16]. After exclusion of these 24 patients, the study cohort was comprised of the 222 subjects with hemophilia or von Willebrand disease with transfusion-induced chronic HCV infections acquired prior to 1988 and followed for 6,117 person years (py) (Table I). 122 were HIV+ and 100 were HIV-. 9 HIV+ and 1 HIV- were coinfected with hepatitis B. Median ages were 29 yrs at enrollment and 45.9 yrs at the end of follow-up. Median duration of HCV infection at last visit was 28 yrs, with an interquartile range of 19-35 yrs. 48 (22%) developed thrombocytopenia defined as a platelet count < 100K. 111 out of 222 subjects were deceased. Cause of death was HIV (58), ESLD (15) one of whom developed hepatocellular carcinoma, bleeding (16), other (13) and unknown or missing (9).
Table I. Characteristics of 222 Subjects with Chronic HCV Infections.
| All = 222 | HIV+ = 122 | HIV- = 100 | |
|---|---|---|---|
| Mean age in years (range) at | |||
| HCV Infection | 16.5 (0-65) | 16 (0-53) | 17 (0-65) |
| Study Entry | 29 (1-81) | 28 (4-72) | 29.4 (1-81) |
| End of Follow-up | 45.9(14.38) | 41.7(13.17) | 51(14.2) |
| Sex | |||
| Male | 212 | 122 | 90 |
| Female | 10 | 0 | 10 |
| Race/Ethnicity | |||
| White | 207 | 115 | 92 |
| Black | 4 | 1 | 3 |
| Other | 11 | 6 | 5 |
| Coagulopathy type | |||
| Hemophilia | 208 | 119 | 89 |
| Von Willebrand's disease | 14 | 3 | 11 |
| Hemophilia Treatment | |||
| Concentrate before 1987 | 177 | 114 | 63 |
| Cryo or FFP only | 35 | 2 | 33 |
| Cryo before concentrate | 10 | 6 | 4 |
| Duration of Infection at last visit/death | |||
| Range | 5-53 | 5-53 | 5-48 |
| Median | 28 | 21 | 31 |
| Interquartile range | 19-35 | 16-32 | 27-36 |
| Sum | 6117 | 2996 | 3121 |
| Status | |||
| Deceased | 111 | 90 | 21 |
| Active | 72 | 17 | 55 |
| Lost to follow-up | 39 | 15 | 24 |
| Follow-up time since enrollment | |||
| Range | 0-30.4 | 0.1-30.4 | 0-30.4 |
| Median | 12.7 | 8.6 | 19.6 |
| Interquartile range | 6-26.2 | 3.9-18.5 | 12.5-28.6 |
| Sum | 3308 | 1422 | 1886 |
| Cause of Death (n = 111) | |||
| HIV | 58 | 58 | 0 |
| ESLD | 15 | 8 | 7 |
| Bleeding | 16 | 11 | 5 |
| Other | 13 | 6 | 7 |
| Unknown or missing | 9 | 7 | 2 |
72 subjects were active when data were censored as of 5/15/2015. 16 (22%) had thrombocytopenia, 14 (19%) had evidence of advanced liver disease manifested by ESLD (n=6), stigmata of cirrhosis and portal hypertension on CT scan (n=3), non-bleeding esophageal varices (n=1), or prothrombin times > 3 sec above the upper limit of normal with thrombocytopenia (n=4). 7 were HIV+ and 7 were HIV-.
ESLD
22(10%) developed ESLD by 40 years after HCV infection date (Fig 1). Seven subjects had liver transplants. Cumulative incidence was 12.3% for all subjects at 40 years since infection ( online supplementary material, Fig. 1). Cumulative incidence was 10.1% for HIV+ (n=10) and 13.4% for HIV- (n=12) subjects. 14/72 (19%) of active subjects had evidence of ESLD or advanced liver disease on imaging or with laboratory evidence of thrombocytopenia with impaired hepatic function as defined above.
Fig 1. Time from HCV infection to development of ESLD in 22 individuals.
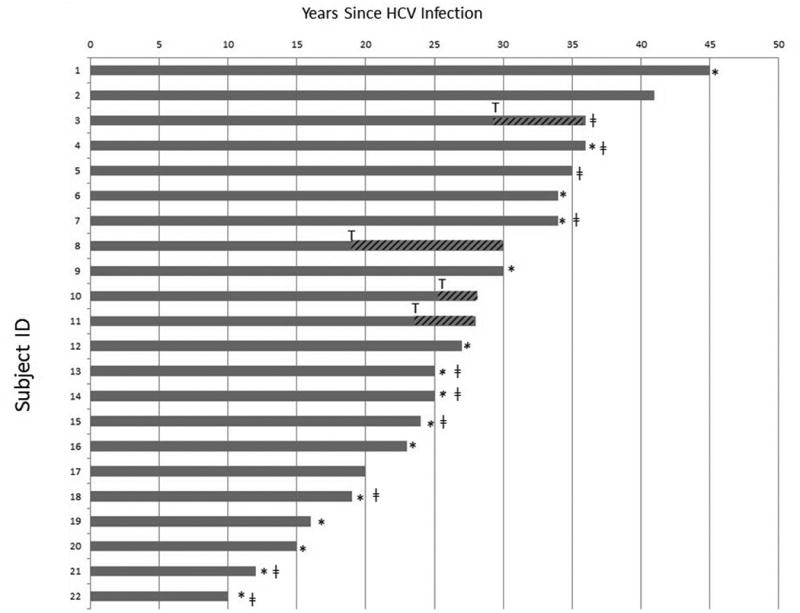
*Denotes time of death. ǂ denotes HIV positive. T denotes time of liver transplant.
Survival
Overall survival for the entire cohort of 222 subjects 40 years after HCV infection date was 45.5%. HIV positive subjects had a significantly shorter survival time than those who were HIV negative ( online supplementary material, Fig. 2). Those who were infected with HCV only had an overall survival of 75.2% 40 years after infection. This was comparable to the 40 year survival rate of 83% in the subset of 65 subjects in the MHCS II study with imputed dates of infection (data not shown). Those who were co-infected with HIV had an overall survival of only 24% at 40 years after HCV infection (P<.001)
Overall survivals were age dependent. Those infected under age 15 had a survival probability of 92% at 40 years compared to 54% for those infected over age 30 (P=0.014) (Fig 2).
Fig 2. Overall survival probability by age group for HIV- individuals.
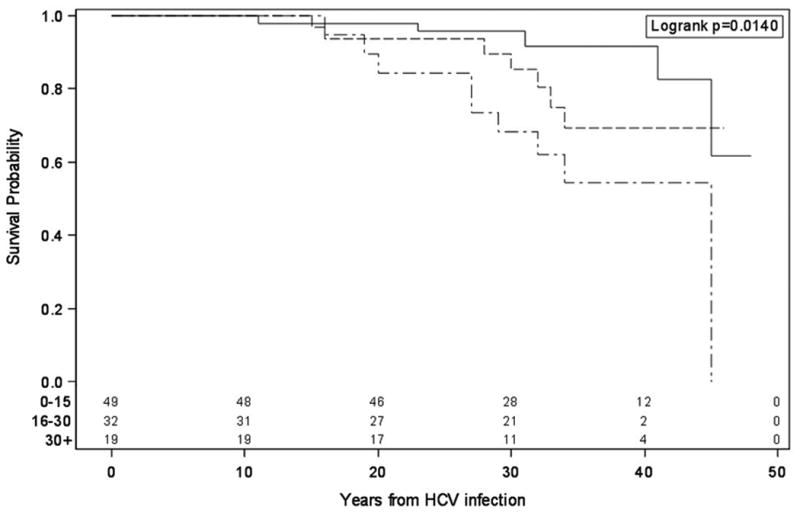
Top curve – individuals infected between 0 and 15 years of age. Middle curve – individuals infected between 16 and 30 years of age. Bottom curve – individuals infected over 30 years of age. Data are computed according to the Kaplan-Meier survival curve technique.
Cause specific mortality
The cumulative incidence of deaths from HIV escalated sharply during the early years of the HIV epidemic, and then leveled off after 35 years at 29% with the advent of active retroviral therapy (ART) in the mid-90s (Fig 3). Deaths from ESLD continued to increase slowly but steadily during this time to 8.3% by 40 years after HCV infection. Deaths from bleeding increased slightly faster to 8.8% by year 40. The trend after 40 years of HCV infection was higher for deaths from bleeding than from ESLD, suggesting that bleeding deaths may have been confounded by the development of thrombocytopenia secondary to portal hypertension.
Fig 3. Cumulative incidence of cause specific deaths since HCV infection for overall cohort.
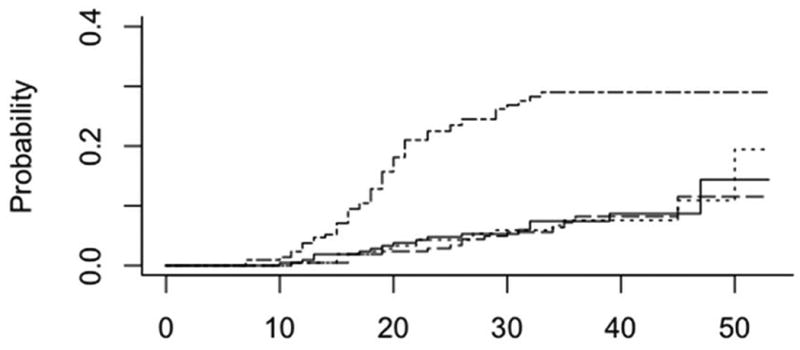
HIV (top curve), ESLD (- - - dashed line), Bleeding (solid line) Other (….. dotted line)
The cumulative incidence of deaths from ESLD increased 10 years after HCV infection in a similar pattern in both HIV negative and HIV positive subjects, reaching 8.1% and 7.8% respectively by 40 years.
For the entire cohort, the annual hazard rate for deaths from HIV rose steeply to a peak of 0.015 cases per year (1.5/100py) by 20 years after HCV infection and dropped sharply to 0.002 (0.2/100py) by 40 years, while the rates for ESLD and bleeding rose more slowly and then leveled off after 30 yrs to .003 (0.3/100 py) for ESLD and .004 (0.4/100py) for bleeding (online supplementary material, Fig. 3).
For the HIV negative subjects, the annual hazard for death for ESLD and bleeding was near zero for the first 15 yrs, and then rose slowly but steadily over the next 20 yrs, to 0.4 /100 py for ESLD and 0.2/100 py for bleeding. This was not significantly different from the HIV positive subjects by 30 yrs (data not shown).
Treatment response rates
60 of the 184 subjects who were found to be positive for HCV RNA when testing became available in the early 1990s have completed 79 treatment episodes with one or more anti-viral regimens. 51/60 eventually achieved a sustained viral response (SVR) with one or more courses of therapy. Overall response rates were divided into three time periods - 1990-2001, 2002-2011, and 2012 through 5/21/15 (online supplementary material, Table 1). During the first period, 7/21 (33%) achieved SVRs 6 mos following treatment to standard interferon (IFN). During the second period, 17/29 (59%) achieved SVRs with pegylated interferon (PEG IFN) alone or in combination with ribavirin (n= 15), or with an NS3a/4a protease inhibitor in combination with PEG IFN and ribavirin (RBV) (n=1) Since 2012, 27/29 (93%) including all 10 HIV positive subjects achieved SVRs 12 weeks following treatment with one of the following direct acting antiviral (DAA) regimens.
NS3/4a protease inhibitor with PEG IFN and RBV
NS5b polymerase inhibitor with PEG IFV and RBV
Combination of NS3/4a protease inhibitor & NS5b polymerase inhibitor
Combination of NS5b polymerase inhibitor with RBV
Combination of NS5b polymerase inhibitor with NS5a inhibitor.
One responder received only PEG IFN plus RBV. One failure did not complete his prescribed 12 week course of therapy. The other did not receive a DAA. All 27, including 3 with ESLD and all 10 HIV+ subjects who completed the prescribed course of treatment which included a DAA achieved SVRs with combination DAA therapy.
One hundred six of the 184 HCV RNA positive subjects were genotyped. Fifty-nine were type l, 17 type 2, 23 type 3, 4 type 4 and 3 mixed genotypes. During the first period, 0/11 type 1 and 2/3 type 2/3 subjects achieved SVRs. During the second period, 6/14 (43% type 1 and 9/11 (82%) type 2/3 had SVRs. During the most recent time period, all 20 genotype 1 subjects and all 6 type 2/3 who completed treatment had SVRs.
Discussion
The course of chronic HCV infections is highly variable [4]. Most persons have relatively mild disease with slow progression over many years. However, progressive HCV infection carries significant morbidity and mortality, and is a major cause of cirrhosis and ESLD estimated to occur in 15-25% over 20 to 30 years of infection [5-9, 17-22]. Difficulties in identifying suitable cohorts with recognized acute infections, and the long follow-up time required to reach meaningful endpoints in untreated individuals are major barriers to defining the natural history of chronic HCV infections.
The vast majority of acute HCV infections in persons with hemophilia occurred between 1965 and 1985 [15]. Although serological tests for HCV were not available until 1990-92, individual infection dates can be reasonably estimated as the date of first exposure to blood products prior to the advent of effective viral inactivation techniques in the mid-1980s. Since many persons with hemophilia are followed at regular intervals at hemophilia treatment centers throughout their lifetimes, this group of individuals presents a unique opportunity to study the natural history of chronic HCV infections over several decades before effective treatment modalities with the DAAs became available.
In our cohort of 222 persons with hemophilia followed since 1973, 22(10%) developed ESLD. Cumulative incidence of ESLD was 12.3% and actuarial survival was 45.5% 40 years after HCV infection date. 122 HIV co-infected persons had a significantly shorter survival of 24%, compared to the 75% survival in the 100 who were HIV negative. Survival was significantly longer for those who were infected at a younger age for both HIV positive and negative individuals. Longer survivals in those infected at a younger age have been previously reported [10, 23-26].
For the overall cohort, the cumulative incidence of deaths from HIV escalated sharply during the early years of the HIV epidemic, and then leveled off after 40 years at 29% with the advent of combination therapy with antiretroviral agents in the mid-1990s. Deaths from ESLD continued a slow steady increase during this time to 8.1% in the HIV negative group and 7.8% in the HIV positive group by 40 years after HCV infection. Deaths from bleeding increased even more to 8.8% by year 40.
The annual hazard rate for deaths from HIV rose steeply to a peak of 1.5/100py by 20 years after HCV infection and then fell precipitously after 30 years, while the rates for ESLD and bleeding rose more slowly and then leveled off after 30 years to 0.3/100 py for ESLD and 0.4/100py for bleeding. For the HIV negative persons, the annual hazard for death for ESLD and bleeding was very low for the first 15 years, and then rose slowly but steadily to 0.4/100 py for ESLD and 0.2/100 py for bleeding by 30 years after infection. This pattern suggests that bleeding may have been confounded by the development of thrombocytopenia secondary to portal hypertension with progressive liver disease.
Several cohort studies have described rates of development of cirrhosis and liver failure in hemophiliacs infected with HCV for 20-30 years [10,23-29], but large studies of survival with high quality data and rigorous documentation of infection followed 30 years or longer are limited to two reports of the same cohort [10, 28]. In an international multicenter cohort study of 687 untreated patients with chronic HCV infected between 1961 and 1990 and followed until August 2005 at 2 hemophilia treatment centers in the UK and one in the Netherlands [10], Posthower et al reported that the cumulative incidence of ESLD 35 years after HCV infection was 11.5% in HIV negative patients, similar to our findings. However, the cumulative incidence of ESLD in HIV positive patients was 35%, compared to only 10% in our study. This discrepancy could be related to frequency of earlier HIV-related deaths in our cohort, selection criteria, or sample size. Independent risk factors were HIV coinfection, older age at infection, alcohol abuse and presence of HCV genotype 1. Six years later when 30% of these patients had a follow up of >35 years, van de Putte et al reported that ESLD occurred in 90/700 (13%) with chronic HCV and in 88/510 (17%) without successful antiviral therapy a median of 31 years after infection [28]. Importantly, 22 patients developed HCC and 9 new cases (4%) were diagnosed after the 2005 evaluation 6 years earlier. By way of comparison, in our cohort, 22/222 (10%) with chronic HCV developed ESLD, including 1 with HCC, a median of 28 years after HCV infection.
In a cohort study of mortality from liver cancer and liver disease in 4865 hemophilic men and boys in the UK treated between 1969 and 1985 and followed until 1993, Darby et al [24] reported that the cumulative risk of death based on death certificate information 25 years after first exposure to clotting factor was 1.4% at all ages and 14.3% for those whose first recorded concentrate was at age 45 or older. The risk was four times higher for HIV positive than for HIV negative patients. In a study of 138 HCV infected patients from Sheffield in the UK followed up to 28 years, Makris et al [25] found that the incidence of biopsy proven cirrhosis rose rapidly 15 years after HCV infection to 15.6 per 1000 py, and that the duration of infection and age at infection were independently associated with the development of both cirrhosis and liver failure. In an Italian cohort of 88 HIV-HCV infected hemophiliacs, Franchini et al reported that 18 (20%) had severe liver disease or cirrhosis, and 4 (4.5%) had hepatic decompensation after a follow-up period of 25 years, with 3 (3.4%) liver related deaths [27]. In another Italian cohort of 356 HIV negative persons with von Willebrand disease and 340 HIV negative persons with hemophilia A or B, Federici et al reported that the cumulative incidence of advanced liver disease was 11% and 10% respectively [29].
Since the first report of an increased frequency of severe liver disease among HIV coinfected persons with HCV [30], numerous studies have reported an accelerated progression of ESLD in HIV co-infected PWH. Yee et al [26] reported that progression to liver-related deaths 25 years after exposure to HIV was 21% compared to 3% in HIV negative PWH. Ragni et al [12] found that the risk of ESLD was significantly greater among HIV positive than among HIV negative PWH (RR 3.72) and increased with each decade of HCV and HIV infection (RR 3.72). Goedert et al [11] reported an annual hazard rate of ESLD after 16 years of 14% in HIV positive and 2.6% in HIV negative PWH with chronic HCV infections. In the sub- cohort of 271 PWH chronically infected with HCV from the Royal Free Hospital in London included in the Posthower study, Yee et al reported that the progression rate to liver related death 25 years after exposure to HCV was 19% [26]. After 13 years from 1985, progression rates to death from those who were HIV positive were 57% compared to 8% for those who were HIV negative.
Our study confirms the markedly increased risk of death from ESLD in HIV co-infected PWH prior to the advent of combination antiretroviral therapy in the mid-1990s. It also emphasizes an age-related but very slow continued progression to ESLD in HIV negative PWH. For those who were HIV negative, cumulative survival at 40 years was 90% for those infected under age 15 yrs compared to 50% for those infected over 30 yrs of age. For our 100 HIV negative PWH, there were 7 (7%) ESLD related deaths infected with HCV a median of 31 years, compared to 3/88 HIV negative (3.4%) infected with HCV for a median of 25 yrs at 3 Italian Hemophilia centers [27], and 3% of liver related deaths after 25 years of HCV exposure in the HIV negative PWH in the Yee study [26].
Our study points out the increased hazard of fatal bleeding events associated with liver disease progression in both HIV positive and HIV negative PWH. Portal hypertension with the development of thrombocytopenia increases the bleeding risk and adds more urgency to treatment of longstanding HCV infections in persons with hemophilia.
Treatment
An understanding of the natural history of chronic HCV infection is necessary for decision making regarding the urgency for treatment to limit the development of cirrhosis and HCC now that highly effective DAAs are achieving SVRs of >95% of patients with chronic HCV.
The first alpha IFN for the treatment of HCV was approved in 1991. Combination treatment with alpha IFN and RBV, approved in 1998, was followed by PEG IFN in combination with RBV from 2001-2010, during which time SVRs rose from approximately 15% to 50%. SVRs were genotype dependent, with <50% of those with genotype 1, the most common genotype, responding, compared to >80% of those with genotypes 2 and 3 [31].
In May 2011, the FDA approved the first class of DAAs (NS3/4a protease inhibitors) for use with PEG IFN and RBV as triple therapy. While the SVR rates dramatically improved to 60-80%, therapy was still complicated by a cumbersome treatment protocol with numerous side effects, including, but not limited to, rash, anemia, thrombocytopenia and fatigue [32].
Antiviral therapy rapidly evolved in the following three years with the approval of new DAA's which no longer required the use of interferon, were easy to administer and had minimal side effects. Specifically these included:
the second generation of NS3/4a protease inhibitors (simeprevir, pariteprevir, and grazoprevir in November 2013, December 2014, and January 2015, respectively);
the first NS5b polymerase inhibitor (sofosbuvir in December 2013);
the first NS5a inhibitors (ledipasvir, ombitasivr, daclatasvir and elbasvir in October 2014, December 2014, July 2015, and January 2016, respectively);
the first NS5rb non-nucleoside polymerase inhibitor (dasabuvir in December 2014).
With these DAAs, SVR rates exceeding 95% were routinely reported in well compensated patients across genotypes [33-38]. In our study, 60 subjects (27%) underwent 79 treatment episodes. 51/60 eventually achieved an SVR with one or more courses of treatment. Response rates improved dramatically from 33% who received standard IFN , to 58% who received PEG IFN plus RBV, to all 27 of those who completed a course of therapy with combination DAAs, including 10/10 HIV positive individuals with genotype 1 and 3 with ESLD.
Study Limitations
One limitation of our study is the inclusion of a 9 individuals who were co infected with HBV as well as those who may have abused alcohol. However, the inclusion of these subjects would have biased our results toward shorter rather than longer survival.
Another limitation is the lack of liver biopsies, autopsy histology or fibroscan measurements. Had these been available, the number of patients with cirrhosis is likely to have been highter. A third limitation is the inclusion of subjects who were treated for their HCV infections. However, 73% received no treatment, and only 24 (11%) achieved SVRs with IFN based treatments prior to 2012. Therefore, the effect of treatment bias on 40-year survival estimates is minimal.
A major strength of our study is that by using patient reported dates of first exposure to blood products recorded on all patients followed prospectively since 1973, we have minimized selection and survival bias.
Conclusions
In a cohort of 222 persons with hemophilia and von Willebrand disease with chronic HCV followed at yearly intervals from 1973-2015, 10% developed ESLD. After 40 years of infection, cumulative incidence of ESLD was 12.3%, and actuarial survival was 45.5%. Survival was significantly longer for those who were HIV negative and those who were infected at a younger age. Cause specific deaths from ESLD and bleeding were 8.8% and 8.3%respectively. All 27 persons treated after 2011 with a full course of DAAs achieved an SVR. These findings help to elucidate the very long natural history of HCV infections with hemophilia. Importantly, they emphasize the need for aggressive surveillance for the detection of early fibrosis to reduce the risk of development of ESLD and associated bleeding events with increasing age now that curative treatment is possible in >95% of individuals.
Supplementary Material
Online Supplementary Data:
Fig 1. Overall cumulative incidence of ESLD in 222 individuals with chronic hepatitis C virus infection.
Fig 2. Overall survival probability by HIV status since HCV infection. Data are computed according to the Kaplan-Meier survival curve technique, with HCV infection dates calculated from the estimated dates of exposure to contaminated blood products in 100 HIV- (solid line) and 122 HIV+ individuals ( dashed line). Numbers indicate numbers of patients in follow up at each 10 year time point.
Fig 3. Cause specific hazard rates of death for the overall cohort. HIV (top curve) ESLD (----- dashed line), Bleeding (solid line), Other (….. dotted line).
Acknowledgments
The authors gratefully acknowledge the assistance of Gail Long for data management, Lisa Baker, RN, for supportive care, and Linda Nelson for manuscript preparation.
This project was partially supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This project was also partially supported by a gift from The Livingston Trout & Mellinger Medical Research Foundation
Footnotes
Author contributions: Each author contributed significantly to the manuscript and are in agreement with its content. The following contributions were made:
MEE conceived of the study and wrote the manuscript.
IRS contributed to the writing of the manuscript and participated in the treatment of the study subjects.
LK designed and oversaw the statistical analysis, wrote the statistical method section and critically reviewed the manuscript.
ML performed the statistical analysis and reviewed the interpretation of the results..
All authors had access to the data set.
Conflict of Interest: None of the authors has any conflicts of interest and no competing financial interests.
References
- 1.Fletcher ML, Trowell JM, Craske J, et al. Non-A, non-B hepatitis after transfusion of factor VIII in infrequently treated patients. Br Med J (Clin Res Ed) 1983;287:1754–1757. doi: 10.1136/bmj.287.6407.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kernoff PB, Lee CA, Karayiannis P, Thomas HC. High risk of non-A non-B hepatitis after a first exposure to volunteer or commercial clotting factor concentrates: effects of prophylactic immune serum globulin. Br J Haematol. 1985;60:469–479. doi: 10.1111/j.1365-2141.1985.tb07444.x. [DOI] [PubMed] [Google Scholar]
- 3.Watson HG, Ludlam CA, Rebus S, et al. Use of several second-generation serological assays to determine the true prevalence of hepatitis C virus infection in haemophiliacs treated with non-virus inactivated factor VII and IX concentrates. Br J Haematol. 1992;80:514–518. doi: 10.1111/j.1365-2141.1992.tb04566.x. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LB. The history of the “natural history” of hepatitis C (1968-2009) Liver Int. 2009;29(s1):89–99. doi: 10.1111/j.1478-3231.2008.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thein H-H, Yo Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–438. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 6.Lingala S, Ghany M. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44:717–734. doi: 10.1016/j.gtc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 8.DiBisceglie AM. Natural history of hepatitis C: Its impact on clinical management. Hepatology. 2000;31:1014–1018. doi: 10.1053/he.2000.5762. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–938. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 10.Posthower D, Makris M, Yee TT, et al. Progression to end-stage liver disease in patients with inherited bleeding disorders and hepatitis C: an international, multicenter cohort study. Blood. 2007;109:3667–3671. doi: 10.1182/blood-2006-08-038349. [DOI] [PubMed] [Google Scholar]
- 11.Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 12.Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 13.Eyster ME. Coping with the HIV epidemic 1982-2007: 25-year outcomes of the Hershey Haemophilia Cohort. Haemophilia. 2008;14:697–202. doi: 10.1111/j.1365-2516.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 14.Eyster ME, Gail MH, Ballard JO, et al. Natural history of human immunodeficiency virus infections in hemophiliacs: Effects of T-cell subsets, platelet counts, and age. Ann Intern Med. 1987;107(1):1–6. doi: 10.7326/0003-4819-107-1-1. [DOI] [PubMed] [Google Scholar]
- 15.Goedert JJ, Chen BE, Preiss L, et al. for the Second Multicenter Hemophilia Cohort Study. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940-1990. Am J Epidemiol. doi: 10.1093/aje/kwm030. doi:2007;10.1093/aje/kmw030. [DOI] [PubMed] [Google Scholar]
- 16.Eyster ME, Sanders J, Goedert JJ. Viral clearance occurs very early during the natural resolution of hepatitis C virus infection in persons with haemophilia. Haemophilia. 2004;10:75–80. doi: 10.1046/j.1351-8216.2003.00836.x. [DOI] [PubMed] [Google Scholar]
- 17.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 18.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 19.Liang TJ, Rehermann B, Seeff LB, Hoffnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 21.Westbrook J, Dusheiko G. Natural history of hepatitis C. Hepatology. 2014;61:S58–68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 23.Telfer P, Sabin C, Devereux H, et al. The progression of HCV-associated liver disease in a cohort of haemophilic patients. Br J Haematol. 1994;87:555–561. doi: 10.1111/j.1365-2141.1994.tb08312.x. [DOI] [PubMed] [Google Scholar]
- 24.Darby SC, Ewart DW, Giangrande PL, et al. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. Lancet. 1997;350:1425–431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 25.Makris M, Preston FE, Rosendaal FR, et al. The natural history of chronic hepatitis C in haemophilics. Brit J Haematol. 1996;94:746–752. doi: 10.1046/j.1365-2141.1996.02343.x. [DOI] [PubMed] [Google Scholar]
- 26.Yee T, Griffioen A, Sabin C, et al. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut. 2000;47(6):845–851. doi: 10.1136/gut.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchini M, Rossetti G, Tagliaferri AC, et al. The natural history of chronic hepatitis C in a cohort of HIV-negative Italian patients with hereditary bleeding disorders. Blood. 2001;98:1836–1841. doi: 10.1182/blood.v98.6.1836. [DOI] [PubMed] [Google Scholar]
- 28.van de Putte DEF, Makris M, Fischer K, et al. Long-term follow-up of hepatitis C infection in a large cohort of patients with inherited bleeding disorders. J Hepatology. 2014;60:39–45. doi: 10.1016/j.jhep.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Federici AB, Santagostino E, Rumi MG, et al. The natural history of hepatitis C virus infection in Italian patients with von Willebrand's disease: a cohort study. Haematologica. 2006;91:503–508. [PubMed] [Google Scholar]
- 30.Eyster ME, Diamondstone LS, Lien JM, et al. for the Multicenter Hemophilia Cohort Study. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of coinfection with human immunodeficiency virus. JAIDS. 1993;6:602–6.10. [PubMed] [Google Scholar]
- 31.Yasin T, Riley TR, Schreibman IR. Current treatment of choice for chronic hepatitis C infection. Infect Drug Resist. 2011;4:11–18. doi: 10.2147/IDR.S4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manzano-Robleda Mdel C, Ornelas-Arroyo V, Barrientos-Gutiérrez T, et al. Boceprevir and telaprevir for chronic genotype 1 hepatitis C virus infection. A systematic review and meta-analysis Ann Hepatol. 2015;14(1):46–57. [PubMed] [Google Scholar]
- 33.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 34.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 35.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 36.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 37.Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 38.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplementary Data:
Fig 1. Overall cumulative incidence of ESLD in 222 individuals with chronic hepatitis C virus infection.
Fig 2. Overall survival probability by HIV status since HCV infection. Data are computed according to the Kaplan-Meier survival curve technique, with HCV infection dates calculated from the estimated dates of exposure to contaminated blood products in 100 HIV- (solid line) and 122 HIV+ individuals ( dashed line). Numbers indicate numbers of patients in follow up at each 10 year time point.
Fig 3. Cause specific hazard rates of death for the overall cohort. HIV (top curve) ESLD (----- dashed line), Bleeding (solid line), Other (….. dotted line).


